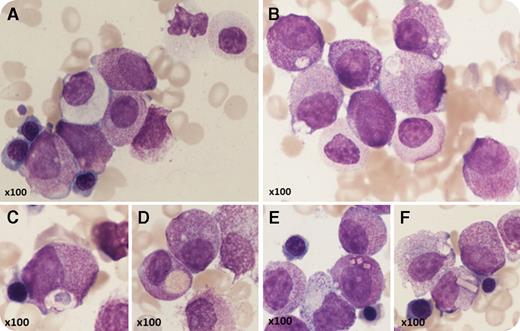
A 53-year-old man with Crohn disease and macrocytic anemia had vitamin B12 deficiency but did not improve on replacement therapy. He developed leukocytosis and his blood count showed: hemoglobin, 87 g/L; white blood cells, 15.4 × 109/L; neutrophils, 2.8 × 109/L; and platelets, 239 × 109/L. Blood film examination showed pseudo–Pelger-Huët neutrophils and abnormal promyelocytes. On bone marrow aspirate (May-Grünwald-Giemsa stain), the majority of cells were abnormal promyelocytes, confirming the diagnosis of acute promyelocytic leukemia (APL); however, morphology was atypical, with round, nonlobulated nuclei and hypergranulation of most cells but no Auer rods (panel A). There was dyserythropoiesis, granulocytic dysplasia (panel A), and nearly absent megakaryocytes. Some promyelocytes were completely agranular (panel A) but many had abnormal inclusions (panels B-F) including crystalline structures (panels E-F). Immunophenotyping showed CD34−, CD117+, CD13+, CD33+, CD56+, HLA-DR−/dim, and myeloperoxidase strong positive. G-banded analysis showed 46,XY, t(11;17)(q23;q21) with del(5)(q22q35). Fluorescence in situ hybridization studies confirmed retinoic acid receptor α (RARA) (17q21) locus (F610/16) rearrangement, and molecular testing confirmed ZBTB16-RARA RNA fusion transcripts. The patient developed a coagulopathy and was resistant to all-trans retinoic acid but achieved a morphological and complete molecular remission with daunorubicin/cytarabine.
This is a rare case of ZBTB16-RARA translocation, classified as “acute myeloid leukemia with a variant RARA translocation.” The distinctive morphological features predict a nonpromyelocytic leukemia translocation with RARA. To our knowledge, the crystalline inclusions have not been previously described.
A 53-year-old man with Crohn disease and macrocytic anemia had vitamin B12 deficiency but did not improve on replacement therapy. He developed leukocytosis and his blood count showed: hemoglobin, 87 g/L; white blood cells, 15.4 × 109/L; neutrophils, 2.8 × 109/L; and platelets, 239 × 109/L. Blood film examination showed pseudo–Pelger-Huët neutrophils and abnormal promyelocytes. On bone marrow aspirate (May-Grünwald-Giemsa stain), the majority of cells were abnormal promyelocytes, confirming the diagnosis of acute promyelocytic leukemia (APL); however, morphology was atypical, with round, nonlobulated nuclei and hypergranulation of most cells but no Auer rods (panel A). There was dyserythropoiesis, granulocytic dysplasia (panel A), and nearly absent megakaryocytes. Some promyelocytes were completely agranular (panel A) but many had abnormal inclusions (panels B-F) including crystalline structures (panels E-F). Immunophenotyping showed CD34−, CD117+, CD13+, CD33+, CD56+, HLA-DR−/dim, and myeloperoxidase strong positive. G-banded analysis showed 46,XY, t(11;17)(q23;q21) with del(5)(q22q35). Fluorescence in situ hybridization studies confirmed retinoic acid receptor α (RARA) (17q21) locus (F610/16) rearrangement, and molecular testing confirmed ZBTB16-RARA RNA fusion transcripts. The patient developed a coagulopathy and was resistant to all-trans retinoic acid but achieved a morphological and complete molecular remission with daunorubicin/cytarabine.
This is a rare case of ZBTB16-RARA translocation, classified as “acute myeloid leukemia with a variant RARA translocation.” The distinctive morphological features predict a nonpromyelocytic leukemia translocation with RARA. To our knowledge, the crystalline inclusions have not been previously described.
For additional images, visit the ASH IMAGE BANK, a reference and teaching tool that is continually updated with new atlas and case study images. For more information visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal