Key Points
Only CD99 on endothelial cells, not on neutrophils, participates in neutrophil extravasation in vivo.
A new function was found for CD99: support of chemokine-induced β2-integrin activation and neutrophil arrest by binding to PILR.
Abstract
CD99 is a crucial regulator of the transmigration (diapedesis) of leukocytes through the blood vessel wall. Here, we report that CD99 acts at 2 different steps in the extravasation process. In agreement with previous antibody-blocking experiments, we found that CD99 gene inactivation caused neutrophil accumulation between venular endothelial cells and the basement membrane in the inflamed cremaster. Unexpectedly, we additionally found that leukocyte attachment to the luminal surface of the venular endothelium was impaired in the absence of CD99. Intravital video microscopy revealed that CD99 supported rapid chemokine-induced leukocyte arrest. Inhibition of leukocyte attachment and extravasation were both solely due to the absence of CD99 on endothelial cells, whereas CD99 on leukocytes was irrelevant. Therefore, we searched for heterophilic ligands of endothelial CD99 on neutrophils. We found that endothelial cells bind to the paired immunoglobulinlike receptors (PILRs) in a strictly CD99-dependent way. In addition, endothelial CD99 was coprecipitated with PILRs from neutrophils that adhered to endothelial cells. Furthermore, soluble CD99 carrying a transferable biotin tag could transfer this tag covalently to PILR when incubated with intact neutrophils. Binding of neutrophils under flow to a surface coated with P-selectin fragment crystallizable (Fc) and intercellular adhesion molecule 1 (ICAM-1) Fc became more shear resistant if CD99 Fc was coimmobilized. This increased shear resistance was lost if neutrophils were preincubated with anti-PILR antibodies. We concluded that endothelial CD99 promotes leukocyte attachment to endothelium in inflamed vessels by a heterophilic ligand. In addition, CD99 binds to PILRs on neutrophils, an interaction that leads to increased shear resistance of the neutrophil attachment to ICAM-1.
Introduction
Neutrophil infiltration into tissues is a hallmark of inflammation and a central step of the innate immune response. Precise regulation of this process is of immense importance, because infections demand a rapid and efficient response, whereas an overshooting invasion of neutrophils can be detrimental. Neutrophil recruitment into sites of tissue insult is regulated by an elegant interplay of selectins, chemokines, and integrins. These players orchestrate the capture, rolling, and arrest of leukocytes along the inner lining of the blood vessel wall; following this, leukocytes often crawl on the endothelial cell surface and exit through the endothelial cell layer, mostly through the junctions between endothelial cells.1-3 After this transmigration step, leukocytes cross through the basement membrane, a process that is mechanistically not well understood and that seems to take longer than transmigration through the endothelial barrier.2
Activation of the β2 integrins LFA-1 and Mac-1 is crucial to the extravasation process and enables neutrophils to arrest via LFA-1 and to crawl and pass (diapedesis process) via Mac-1 through the endothelial barrier. Chemokines presented on the endothelial cell surface and endothelial selectins act in concert to trigger integrin activation on leukocytes.4 Whether additional endothelial cell surface proteins are required to support this process is presently unknown.
CD99 is a highly O-glycosylated type I membrane protein that does not belong to any known protein family and was first identified on human lymphocytic leukemia cells.5 It plays an important regulatory role on T cells6-9 and has also been linked to cancer invasiveness and metastasis. Initially, human CD99 was found to participate as a homophilic adhesion molecule in the transmigration of monocytes through human umbilical vein endothelial cell monolayers.10 Antibodies against CD99 blocked diapedesis, acting subsequently to PECAM-1.10 In mice it was found that antibodies against CD99 impaired T-cell recruitment into inflamed skin11 and extravasation of neutrophils into inflamed peritoneum and cremaster by causing accumulation at the interphase between endothelium and the basement membrane.12-14 In contrast to the results with human CD99, antibodies against mouse CD99 inhibited in vitro diapedesis by blocking CD99 on endothelial cells only, and not on neutrophils. Whether CD99 acts in vivo on both cell types during neutrophil diapedesis has not yet been studied.
The paired immunoglobulinlike receptors α and β (PILR-α and PILR-β) on dendritic cells (DCs) and natural killer (NK) cells represent binding partners for CD99 on lymphocytic cells.15 PILRs are mainly expressed on DCs, NK cells, and myeloid cells.15-17 PILR-α is an inhibitory receptor bearing in its cytoplasmic tail 2 immunoreceptor tyrosine-based inhibitory motifs, and PILR-β has activating functions that require the association with the adaptor molecule DAP12. The PILRs are lectin-like molecules that bind to sialylated O-linked carbohydrate side chains on CD99.18 Gene inactivation of PILR-α causes enhanced neutrophil recruitment into the inflamed peritoneum.19
Here, we have shown that gene inactivation of CD99 impairs leukocyte extravasation at 2 steps of the extravasation cascade: it causes accumulation of leukocytes between the endothelium and the underlying basement membrane, and it attenuates adhesion of leukocytes to the luminal surface of inflamed venules. In addition, we show that only the CD99 on endothelial cells supports leukocyte extravasation, whereas CD99 on leukocytes is irrelevant. Finally, we demonstrate that endothelial CD99 binds on neutrophils to PILR, which modulates the interaction of neutrophils with intercellular adhesion molecule 1 (ICAM-1), thereby enhancing adhesion under flow.
Materials and methods
Animals
CD99−/− mice (C57Bl/6) from Kumamoto University (21-B6T44 clone in Exchangeable Gene Trap Clone database; http://egtc.jp/action/access/clone_detail?id=21-B6T44) were genotyped as described,20 using wild-type (WT) primers, F (5-′CGAGTGACGAC TTCAACCTGGGCG-3′), R (5′-TGAGTCTCCGTGTGGCCT TG-3′), generating a WT band of 917 bp, and mutant primers, F (5′-GCGTTACCCA CCTTAATCG-3′), R (5′-TGT GAG CGA GTA ACA ACC CG-3′), generating a trap-locus band of 320 bp. All experiments were carried out under the German legislation for the protection of animals and were approved by the Landesamt fuer Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen.
Cells
Mouse endothelioma cells bEnd.5,21 and Chinese hamster ovary (CHO) cells (American Type Culture Collection), have been described. CHO cell lines stably expressing various fragment crystallizable (Fc) fusion proteins were generated by electroporation according to established procedures.22 Mouse endothelial cells from lung23 and skin24 were isolated and cultured, and neutrophils were isolated from mouse bone marrow, as described.12
Antibodies
The following were used: affinity-purified rabbit polyclonal antibodies against CD99,12 VE-cadherin (VE42),25 ESAM (VE19),22 JAM-A,26 and rat monoclonal antibody against ESAM (1G8)22 and laminin α5 (4G6)27 ; anti–PILR-α (AF4318), goat anti-mouse MRP14 antibody (S100a) (R&D Systems); anti-PECAM-1 Alexa Fluor 555 (390) (Millipore), anti-PECAM-1 (M20), and anti-ICAM-1 (M19) (Santa Cruz Biotech); donkey anti-rat Alexa Fluor 488, donkey anti-goat Alexa Fluor 568, and donkey anti-rabbit Alexa Fluor 647 (Invitrogen). Polyclonal rabbit antisera against mouse PILR were generated using a PILR-β-Fc and a PILR-α-Fc fusion protein. Antibodies against the immunoglobulin G1 (IgG1) Fc region were removed by depletion with human IgG1 CNBr-activated Sepharose (GE Healthcare, Freiburg, Germany). Specific antibodies against PILR were affinity purified with the immunogen Fc constructs immobilized on CNBr-activated Sepharose (GE Healthcare).
Intravital microscopy
CD99−/− mice were injected intrascrotally with 500 ng tumor necrosis factor α (TNF-α) 2 hours before exteriorization of the cremaster muscle and preparation for intravital microscopy, as described.12,25 For analyzing the duration of crawling and transmigration, Ly-EGFP bone marrow cells were transplanted into WT and CD99−/− mice. Six weeks later, the mice were intrascrotally stimulated with 500 ng TNF-α. Endothelial cell junctions were labeled with 25 μg of Alexa-Fluor-555 PECAM antibody and intravenously injected; this step was followed by preparation of the cremaster as described.12,25 Thirty-minute videos were taken over a period of 1 hour with an LSM 780 confocal microscope (Zeiss) beginning 90 minutes after TNF-α stimulation. Imaris software (Bitplane) was used to track neutrophils.
Bone marrow transplantation
Transplantations were carried out as described.28
Cremaster whole-mount confocal immunofluorescence
Whole mounts of the inflamed murine cremaster muscle were used for immunostaining to analyze the step or position of the arrested leukocytes in the extravasation cascade, essentially as previously described.13
Chemokine-induced arrest
For chemokine-induced arrest studies in cremaster venules, the carotid arteries of anesthetized mice were cannulated and the cremaster was prepared for intravital microscopy. Upon application of 600 ng of CXCL1 through the carotid artery, the number of adherent cells was immediately monitored every minute over a time period of 15 minutes.
Adhesion of endothelial cells to immobilized PILR Fc
Ninety-six well plates (Falcon) were coated with rabbit-α-human F(ab′)2 (15 µg/mL in phosphate-buffered saline [PBS]; Jackson Immuno Research) at 37°C for 1 hour, followed by overnight blocking with 3% bovine serum albumin in PBS with 1 mM CaCl2 and 1 mM MgCl2 (PBS-MC), washing with PBS-MC, and incubating with PILR-α Fc (10 µg/mL), PILR-β Fc (20 µg/mL), or E-selectin Fc (20 µg/mL) in PBS-MC for 2 hours at room temperature. Further steps were performed as previously described.29
Results
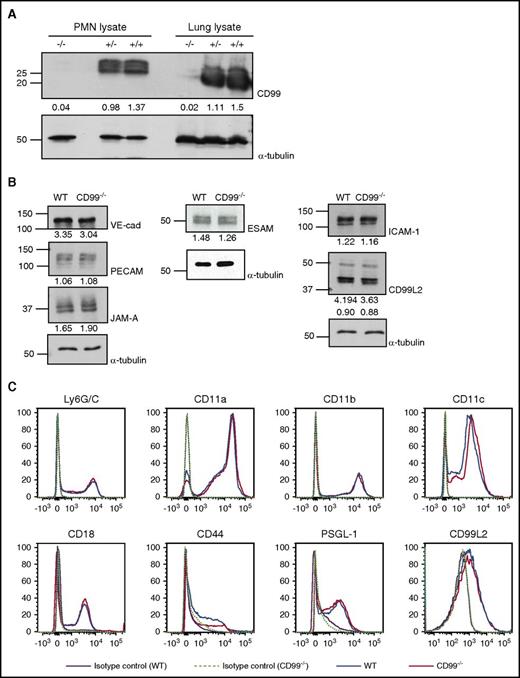
CD99 gene inactivation does not affect the expression of other adhesion molecules relevant for leukocyte extravasation
CD99 gene–inactivated (CD99−/−) mice were generated previously by an exchangeable gene trap method.20 In immunoblots, CD99 was neither detected in lung lysates nor in isolated neutrophils of these mice (Figure 1A). Skin endothelial cells isolated from CD99−/− mice or WT littermates and treated for 16 hours with TNF-α showed similar levels of PECAM-1, ESAM, CD99L2, JAM-A, VE-cadherin, and ICAM-1 in immunoblots (Figure 1B). Likewise, expression levels of CD11a, CD11b, CD11c, CD18, CD44, PSGL-1, and CD99L2 on the surface of neutrophils were similar for both genotypes (Figure 1C). Thus, CD99 gene inactivation does not affect the expression of any of the tested adhesion molecules relevant for leukocyte extravasation.
Expression of adhesion receptors other than CD99 is not affected by CD99 gene inactivation. (A) Detergent lysates of bone marrow–derived neutrophils or complete lungs from WT (+/+), heterozygous (+/−), or homozygous (−/−) CD99 gene–inactivated mice were immunoblotted for CD99 or α-tubulin (as indicated). Band intensities normalized to α-tubulin control are indicated below the blots. (B) Endothelial cells isolated from skins of either WT mice or CD99−/− mice were immunoblotted for the indicated antigens; quantification of the signals was standardized to α-tubulin signals (indicated below). (C) Fluorescence-activated cell sorting analysis of bone marrow–derived polymorphonuclear neutrophils (PMNs) of CD99−/− (red) and WT mice (blue) for the indicated antigens; purple solid lines and light green dotted lines mark antibody-isotype controls for WT and CD99−/− mice, respectively. VE-cad, VE-cadherin.
Expression of adhesion receptors other than CD99 is not affected by CD99 gene inactivation. (A) Detergent lysates of bone marrow–derived neutrophils or complete lungs from WT (+/+), heterozygous (+/−), or homozygous (−/−) CD99 gene–inactivated mice were immunoblotted for CD99 or α-tubulin (as indicated). Band intensities normalized to α-tubulin control are indicated below the blots. (B) Endothelial cells isolated from skins of either WT mice or CD99−/− mice were immunoblotted for the indicated antigens; quantification of the signals was standardized to α-tubulin signals (indicated below). (C) Fluorescence-activated cell sorting analysis of bone marrow–derived polymorphonuclear neutrophils (PMNs) of CD99−/− (red) and WT mice (blue) for the indicated antigens; purple solid lines and light green dotted lines mark antibody-isotype controls for WT and CD99−/− mice, respectively. VE-cad, VE-cadherin.
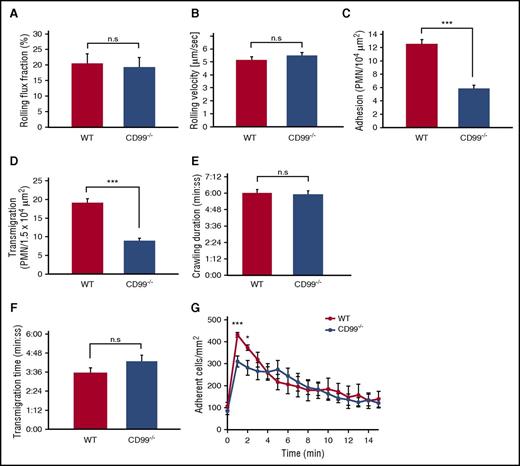
CD99 gene ablation inhibits neutrophil adhesion and extravasation in venules of the inflamed cremaster
It has been shown by our group and others that antibodies against CD99 inhibit leukocyte extravasation in the mouse.11-13,30 To exclude potential indirect effects by the antibodies and to test whether CD99 is indeed required for proper extravasation, we examined neutrophil extravasation by intravital microscopy in the inflamed cremaster muscle of CD99−/− mice. Two hours after intrascrotal injection of TNF-α, we found that extravasation was reduced by 53% (±3.5%) in CD99−/− mice compared with WT littermates (Figure 2D). Although rolling flux fraction and rolling velocity were normal (Figure 2A-B), the number of adhering neutrophils in postcapillary venules of CD99−/− mice was reduced by 53% (±4.0%) (Figure 2C). The inhibition of extravasation was in line with our previous antibody effects.12,13 However, no previous study has observed a role of CD99 in adhesion of neutrophils to the endothelium, and this finding was therefore unexpected.
CD99 deficiency impairs leukocyte adhesion and extravasation in the inflamed cremaster. (A-D) WT mice (red bars) and CD99−/− mice (blue bars) were analyzed by intravital microscopy of cremaster tissue after 2 hours of TNF-α stimulation for (A) rolling flux fraction, (B) rolling velocity, (C) adherent leukocytes, and (D) extravasated leukocytes. Results are displayed as mean ± standard error of the mean (SEM) for 5 animals per group (n = 34 for WT and n = 39 for CD99−/− mice, with n being the number of vessels). **P < .01 and ***P < .001 (supplemental Table 1 shows hemodynamic parameters). (E-F) Confocal intravital microscopy of the cremaster of Ly-EGFP bone marrow–transplanted WT and CD99−/− mice, stimulated intrascrotally with 500 ng of TNF-α for 90 minutes, followed by intravenous injection of Alexa 555–coupled PECAM monoclonal antibody, and captured in 30-minute videos. The tracking tool in the Imaris software (Bitplane) was used to determine (E) duration of intraluminal crawling and (F) transendothelial migration (minutes:seconds) in WT (red) and CD99−/− (blue) mice. Results are displayed as mean ± SEM (n = 8 WT mice, leukocytes = 18; n = 7 CD99−/− mice, leukocytes = 13). Not significant (n.s.), as per Student t test. (G) Chemokine-driven arrest of leukocytes in postcapillary venules of the cremaster was analyzed in WT mice (red dots) and CD99−/− mice (blue dots). Videos were made 15 seconds before and every minute from 1 to 15 minutes after injection of CXCL1 (600 ng) into the carotid artery. Data are shown as mean ± SEM (n = 5 WT mice; n = 6 CD99−/− mice). *P < .05 and ***P < .001; n.s. as per Student t test.
CD99 deficiency impairs leukocyte adhesion and extravasation in the inflamed cremaster. (A-D) WT mice (red bars) and CD99−/− mice (blue bars) were analyzed by intravital microscopy of cremaster tissue after 2 hours of TNF-α stimulation for (A) rolling flux fraction, (B) rolling velocity, (C) adherent leukocytes, and (D) extravasated leukocytes. Results are displayed as mean ± standard error of the mean (SEM) for 5 animals per group (n = 34 for WT and n = 39 for CD99−/− mice, with n being the number of vessels). **P < .01 and ***P < .001 (supplemental Table 1 shows hemodynamic parameters). (E-F) Confocal intravital microscopy of the cremaster of Ly-EGFP bone marrow–transplanted WT and CD99−/− mice, stimulated intrascrotally with 500 ng of TNF-α for 90 minutes, followed by intravenous injection of Alexa 555–coupled PECAM monoclonal antibody, and captured in 30-minute videos. The tracking tool in the Imaris software (Bitplane) was used to determine (E) duration of intraluminal crawling and (F) transendothelial migration (minutes:seconds) in WT (red) and CD99−/− (blue) mice. Results are displayed as mean ± SEM (n = 8 WT mice, leukocytes = 18; n = 7 CD99−/− mice, leukocytes = 13). Not significant (n.s.), as per Student t test. (G) Chemokine-driven arrest of leukocytes in postcapillary venules of the cremaster was analyzed in WT mice (red dots) and CD99−/− mice (blue dots). Videos were made 15 seconds before and every minute from 1 to 15 minutes after injection of CXCL1 (600 ng) into the carotid artery. Data are shown as mean ± SEM (n = 5 WT mice; n = 6 CD99−/− mice). *P < .05 and ***P < .001; n.s. as per Student t test.
To rule out indirect effects that would reduce the number of detectable polymorphonuclear neutrophils in the vessel lumen, we determined the duration of crawling and transendothelial migration by confocal intravital video microscopy. No changes were detected (Figure 2E-F; supplemental Video, available on the Blood Web site).
To further explore the nature of this adhesion effect, we tested whether CD99 would influence chemokine-induced β2-integrin activation that triggers leukocyte arrest in less than a minute. Using intravital microscopy, we documented that intracarotid injection of the chemokine CXCL1-triggered leukocyte arrest in vessels of the cremaster within 1 minute. Observing this over a time span of 15 minutes, we found that in CD99−/− mice, the CXCL1-induced number of arrested leukocytes was reduced by 38% (±6%) in the first minute (Figure 2G). Thus, CD99 supports chemokine-induced arrest of neutrophils in inflamed postcapillary venules in vivo.
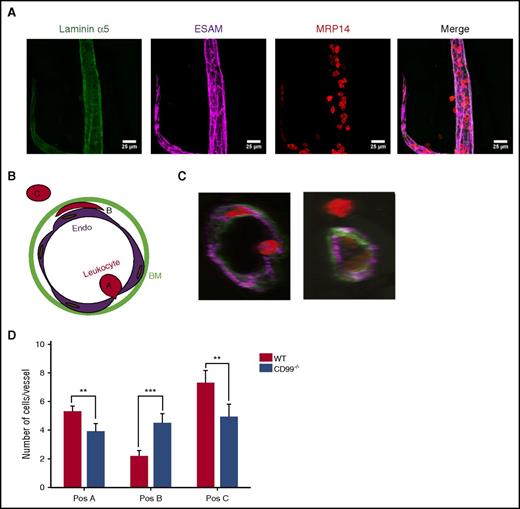
CD99 acts at 2 distinct steps in the extravasation cascade
We have shown previously that anti-CD99 antibodies inhibit leukocyte extravasation by causing accumulation of neutrophils between the endothelium and basement membrane.13 To test whether the absence of CD99 would cause similar effects, we performed whole-mount stainings of the fixed cremaster 4 hours after intrascrotal injection of interleukin-1β. Endothelial cell contacts, neutrophils, and the basement membrane were visualized by staining for ESAM, MRP14, and laminin α5, respectively (Figure 3A). Analysis was performed by confocal microscopy and 3-dimensional image analysis. We found neutrophils at 3 positions in the extravasation pathway: at the apical cell surface (position A), between the endothelium and basement membrane (position B), and fully extravasated (position C) (Figure 3B-C). The number of neutrophils in position B was significantly increased in CD99−/− mice, confirming our previous antibody results (Figure 3D). In addition, we found fewer fully extravasated neutrophils and a reduced number of neutrophils in contact with the endothelium in the vessel lumen. The latter finding is in agreement with our intravital microscopy results (Figure 2C). We concluded that CD99 deficiency reveals, unexpectedly, the contribution of CD99 to 2 steps of the extravasation process: docking to the apical surface of the endothelium, and movement or exit at/from the endothelial cell/basement membrane interphase. In contrast, antibodies against CD99 inhibited only the latter function.13
CD99 gene inactivation affects neutrophil extravasation at 2 steps. Three-dimensional (3D) confocal image analysis of cremaster was used to determine sites of arrest of transmigrating leukocytes in WT and CD99−/− mice. Mice were injected intrascrotally with interleukin-1β (50 ng) for 4 hours, cremaster muscles were prepared from the sacrificed animals, and whole mounts were immunostained for laminin α5 (green), ESAM (purple), and MRP14 (red) to stain the basement membrane, endothelial cells, and leukocytes, respectively. (A) Representative image of a blood vessel used for 3D analysis and evaluation. The first 3 panels show single stainings for the basement membrane (green), endothelium (purple), and leukocyte (red), only, and the last panel shows the merger of all 3 channels. Bars represent 25 µm. (B) Schematic drawing illustrating the criteria used to delineate the 3 positions in which leukocytes are found during extravasation. Position A includes leukocytes that are adhered to the endothelium and usually appear round, position B includes leukocytes which are trapped between the endothelium and the underlying basement membrane and appear flat and oriented parallel to the blood vessel, and position C includes cells which have transmigrated into the tissue. (C) Representative cross-sections of vessels with leukocytes in the 3 positions. (D) Graph presenting the absolute numbers of leukocytes per vessel segment in each of the 3 positions. Data are shown as mean ± standard error of the mean (n = 51 vessels for CD99−/− and n = 52 vessels for WT mice). **P < .01 and ***P < .01, as per Mann-Whitney U test.
CD99 gene inactivation affects neutrophil extravasation at 2 steps. Three-dimensional (3D) confocal image analysis of cremaster was used to determine sites of arrest of transmigrating leukocytes in WT and CD99−/− mice. Mice were injected intrascrotally with interleukin-1β (50 ng) for 4 hours, cremaster muscles were prepared from the sacrificed animals, and whole mounts were immunostained for laminin α5 (green), ESAM (purple), and MRP14 (red) to stain the basement membrane, endothelial cells, and leukocytes, respectively. (A) Representative image of a blood vessel used for 3D analysis and evaluation. The first 3 panels show single stainings for the basement membrane (green), endothelium (purple), and leukocyte (red), only, and the last panel shows the merger of all 3 channels. Bars represent 25 µm. (B) Schematic drawing illustrating the criteria used to delineate the 3 positions in which leukocytes are found during extravasation. Position A includes leukocytes that are adhered to the endothelium and usually appear round, position B includes leukocytes which are trapped between the endothelium and the underlying basement membrane and appear flat and oriented parallel to the blood vessel, and position C includes cells which have transmigrated into the tissue. (C) Representative cross-sections of vessels with leukocytes in the 3 positions. (D) Graph presenting the absolute numbers of leukocytes per vessel segment in each of the 3 positions. Data are shown as mean ± standard error of the mean (n = 51 vessels for CD99−/− and n = 52 vessels for WT mice). **P < .01 and ***P < .01, as per Mann-Whitney U test.
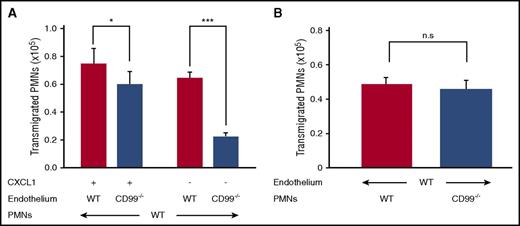
Endothelial CD99 is crucial for neutrophil extravasation, whereas neutrophil CD99 is dispensable
Although CD99 can mediate homophilic interactions of transfected cells,10 we have previously found that anti-CD99 antibodies inhibited neutrophil transmigration in vitro only when incubated with endothelial cells, but not when preincubated with neutrophils.12 To address this issue further, we analyzed the migration of isolated mouse neutrophils through 16-hour TNF-α stimulation of primary lung endothelial cells isolated from either CD99−/− mice or their WT littermates. Transmigration was allowed in the presence or absence of CXCL1. The assay did not distinguish between effects on adhesion or diapedesis. The reduction in transmigration through CD99−/− endothelial cells was 65.4% (±3.97%) without chemoattractant, and 19.7% (±12%) when a chemoattractant was present (Figure 4A). In contrast, neutrophils from CD99−/− mice and WT littermates transmigrated with similar efficiency through WT primary endothelial cells (Figure 4B). Thus, endothelial CD99 is crucial for transmigration in vitro, whereas CD99 on neutrophils is dispensable.
Loss of CD99 from endothelial cells impairs neutrophil transmigration in vitro. (A) Bone marrow–derived neutrophils from WT mice were allowed to transmigrate through 16-hour TNF-α–stimulated, WT (red bars) or CD99−/− (blue bars) primary lung endothelial cells in the presence (30 minutes) or absence (90 minutes) of CXCL1 (n = 24 per group from 4 independent experiments). Results are displayed as mean ± standard error of the mean (SEM). *P < .05 and ***P < .001, as per Mann-Whitney U test. (B) Bone marrow–derived neutrophils from WT mice (red bars) or CD99−/− mice (blue bars), were allowed to transmigrate for 30 minutes through 16-hour TNF-α–stimulated WT primary lung endothelial cells in the presence of CXCL1 (n = 19 per group from 3 independent experiments). Results are displayed as mean ± SEM. n.s., *P < .05, and ***P < .001, as per unpaired Student t test. PMN, polymorphonuclear neutrophil.
Loss of CD99 from endothelial cells impairs neutrophil transmigration in vitro. (A) Bone marrow–derived neutrophils from WT mice were allowed to transmigrate through 16-hour TNF-α–stimulated, WT (red bars) or CD99−/− (blue bars) primary lung endothelial cells in the presence (30 minutes) or absence (90 minutes) of CXCL1 (n = 24 per group from 4 independent experiments). Results are displayed as mean ± standard error of the mean (SEM). *P < .05 and ***P < .001, as per Mann-Whitney U test. (B) Bone marrow–derived neutrophils from WT mice (red bars) or CD99−/− mice (blue bars), were allowed to transmigrate for 30 minutes through 16-hour TNF-α–stimulated WT primary lung endothelial cells in the presence of CXCL1 (n = 19 per group from 3 independent experiments). Results are displayed as mean ± SEM. n.s., *P < .05, and ***P < .001, as per unpaired Student t test. PMN, polymorphonuclear neutrophil.
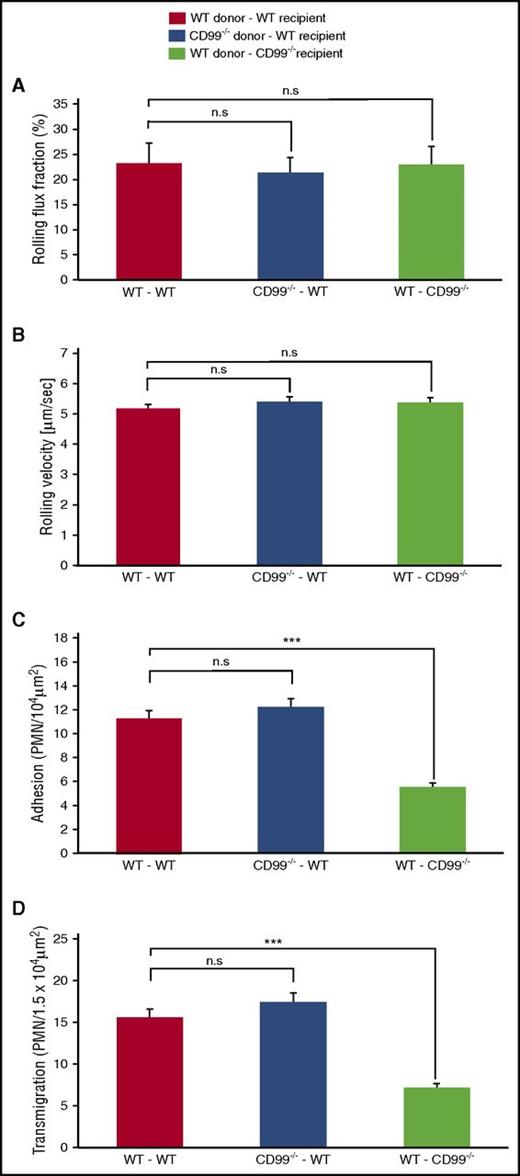
It is not known on which cells, neutrophils or endothelium, CD99 participates in vivo in the process of leukocyte extravasation, because this question cannot be studied with antibodies. To clarify this, we analyzed leukocyte extravasation in the inflamed cremaster of bone marrow–chimeric mice, which lacked the expression of CD99, either on endothelial cells only, or on leukocytes only. CD99−/− mice and WT littermates were lethally irradiated and then transplanted with bone marrow cells from either WT or CD99−/− donor mice, respectively, thereby generating mice which had either WT circulating cells, but CD99-deficient endothelium, or vice versa. Transplantation efficiency was tested by immunoblots of neutrophils from the fully recovered transplanted mice (supplemental Figure 1). For controls, WT donor cells were transplanted into WT recipient mice. Intravital microscopy was performed after 2 hours of TNF-α stimulation. The absence of CD99 on endothelium caused decreased adhesion and transmigration of neutrophils by 49% (±3.3%) and 46% (±2.5%), respectively (Figure 5C-D), as compared with the WT control group. In contrast, the absence of CD99 only on leukocytes had no effect. Rolling flux fraction and rolling velocity were unaffected in either of the 3 groups (Figure 5A-B). Thus, in line with the in vitro transmigration results, only endothelial CD99 is relevant for the extravasation of neutrophils, whereas CD99 on neutrophils is dispensable. This implies that either endothelial CD99 does not interact with CD99 on neutrophils, or this interaction has no relevance for the extravasation process. Furthermore, these results suggest that a heterophilic ligand for endothelial CD99 may exist on neutrophils.
CD99 is required on endothelial cells but not on neutrophils for extravasation from cremaster venules. WT- and CD99−/−-recipient mice were lethally irradiated and transplanted with donor bone marrow as follows: WT donors into WT recipients (red bars), CD99−/− donors into WT recipients (blue bars), and WT donors into CD99−/− recipients (light green bars). Six weeks later, mice were analyzed by intravital microscopy of the cremaster after 2 hours of TNF-α stimulation for (A) rolling flux fraction, (B) rolling velocity, (C) numbers of adherent leukocytes, and (D) extravasated leukocytes (supplemental Table 2 shows hemodynamic parameters). Results are displayed as mean ± standard error of the mean for 5 mice per group, with a total of 35, 33, and 34 vessel segments per group, respectively. ***P < .001 and n.s. as per 1-way analysis of variance. PMN, polymorphonuclear neutrophil.
CD99 is required on endothelial cells but not on neutrophils for extravasation from cremaster venules. WT- and CD99−/−-recipient mice were lethally irradiated and transplanted with donor bone marrow as follows: WT donors into WT recipients (red bars), CD99−/− donors into WT recipients (blue bars), and WT donors into CD99−/− recipients (light green bars). Six weeks later, mice were analyzed by intravital microscopy of the cremaster after 2 hours of TNF-α stimulation for (A) rolling flux fraction, (B) rolling velocity, (C) numbers of adherent leukocytes, and (D) extravasated leukocytes (supplemental Table 2 shows hemodynamic parameters). Results are displayed as mean ± standard error of the mean for 5 mice per group, with a total of 35, 33, and 34 vessel segments per group, respectively. ***P < .001 and n.s. as per 1-way analysis of variance. PMN, polymorphonuclear neutrophil.
Endothelial CD99 binds to PILRs on neutrophils
PILR-α and PILR-β are the only known heterophilic ligands for CD99 to date.15,18 This interaction is based on the lectin function of PILRs, which bind to sialic acid–based carbohydrates on CD99. To test whether PILRs can bind to the glycosylated form of CD99 on endothelial cells, we allowed bEnd.5 endothelial cells to adhere to immobilized PILR-α Fc or PILR-β Fc in 96-well plates. We found that bEnd.5 cells bound specifically to each of the 2 constructs as compared with E-selectin Fc as a negative control (Figure 6A). Specific binding was completely inhibited with anti-CD99 antibodies in a concentration-dependent way (Figure 6A). In addition, inhibition of the expression of CD99 by small-interfering RNA (knockdown efficiency shown in Figure 6C) abolished bEnd.5 binding to PILR-α Fc and PILR-β Fc completely (Figure 6B). We concluded that the PILRs interact with endothelial CD99 as an essential ligand for these receptors on endothelial cells. We cannot exclude whether CD99 might act in cooperation with other endothelial cell surface molecules.
CD99 on EC represents the ligand for PILRs on neutrophils. (A) bEnd.5 cells were either untreated or pretreated with antibodies from preserum or anti-CD99 (as indicated) and subsequently allowed to adhere onto a surface immobilized with E-selectin Fc (control; red bar), PILR-α Fc (blue bars), or PILR-β Fc (light green bars) constructs for 60 minutes at 37°C (n > 17 from 3 independent experiments). (B) bEnd5 cells were pretreated either with control small-interfering RNA (siRNA) or with CD99 siRNA (as indicated), and adhesion assays were performed as described in panel A (n > 16 from 3 independent experiments). (C) Endothelial cells used in panel B were immunoblotted for CD99 or for tubulin (as indicated) after treatment with either control or CD99 siRNA. (D) Polymorphonuclear neutrophils (PMNs) were allowed to adhere to TNF-α–treated primary lung endothelial cells isolated from WT mice or from CD99 knockout mice (as indicated) for 30 minutes at 37°C. The cell mix was then subjected to immunoprecipitation with anti-PILR antibodies or control IgG (as indicated). Immunoprecipitates (left panel) or cell lysates (right panel) were immunoblotted for CD99 and PILR (as indicated). (E) Lysates of mouse primary endothelial cells and PMNs were immunoblotted for CD99. Note the difference in electrophoretic mobility. (F) CD99 Fc or huIgG were labeled with sulfo-N-hydroxysuccinimidyl-2-(6-[biotinamido]-2-[p-azido benzamido]-hexanoamido) ethyl-1,3′-dithioproprionate biotin and separately incubated with PMNs for 1 hour at 37°C, followed by photoactivation (312 nm ultraviolet light) to crosslink with the interacting binding partners for 10 minutes. Lysates were prepared from washed cells and crosslinked biotinylated proteins were immunoprecipitated (IP) by streptavidin and immunoblotted for PILR. Samples were reduced by 100 mM dithiothreitol to complete the biotin transfer. Data in panels D and F are representative of 2 (D) and 3 (F) independent experiments. *P < .05, ***P < .001. Error bars show standard error of the mean. One-way analysis of variance was followed by the Holm-Šídák test for panels A and B.
CD99 on EC represents the ligand for PILRs on neutrophils. (A) bEnd.5 cells were either untreated or pretreated with antibodies from preserum or anti-CD99 (as indicated) and subsequently allowed to adhere onto a surface immobilized with E-selectin Fc (control; red bar), PILR-α Fc (blue bars), or PILR-β Fc (light green bars) constructs for 60 minutes at 37°C (n > 17 from 3 independent experiments). (B) bEnd5 cells were pretreated either with control small-interfering RNA (siRNA) or with CD99 siRNA (as indicated), and adhesion assays were performed as described in panel A (n > 16 from 3 independent experiments). (C) Endothelial cells used in panel B were immunoblotted for CD99 or for tubulin (as indicated) after treatment with either control or CD99 siRNA. (D) Polymorphonuclear neutrophils (PMNs) were allowed to adhere to TNF-α–treated primary lung endothelial cells isolated from WT mice or from CD99 knockout mice (as indicated) for 30 minutes at 37°C. The cell mix was then subjected to immunoprecipitation with anti-PILR antibodies or control IgG (as indicated). Immunoprecipitates (left panel) or cell lysates (right panel) were immunoblotted for CD99 and PILR (as indicated). (E) Lysates of mouse primary endothelial cells and PMNs were immunoblotted for CD99. Note the difference in electrophoretic mobility. (F) CD99 Fc or huIgG were labeled with sulfo-N-hydroxysuccinimidyl-2-(6-[biotinamido]-2-[p-azido benzamido]-hexanoamido) ethyl-1,3′-dithioproprionate biotin and separately incubated with PMNs for 1 hour at 37°C, followed by photoactivation (312 nm ultraviolet light) to crosslink with the interacting binding partners for 10 minutes. Lysates were prepared from washed cells and crosslinked biotinylated proteins were immunoprecipitated (IP) by streptavidin and immunoblotted for PILR. Samples were reduced by 100 mM dithiothreitol to complete the biotin transfer. Data in panels D and F are representative of 2 (D) and 3 (F) independent experiments. *P < .05, ***P < .001. Error bars show standard error of the mean. One-way analysis of variance was followed by the Holm-Šídák test for panels A and B.
To test whether PILR expressed on neutrophils is able to bind to CD99 expressed on endothelial cells, we attempted to coimmunoprecipitate endothelial CD99 with anti-PILR antibodies from neutrophils adhering to endothelial cells. For these experiments, we generated affinity-purified rabbit antibodies against PILR-α-Fc and PILR-β-Fc fusion proteins. Specificity and reactivity were tested on transfected CHO cells and on neutrophils (supplemental Figure 2). Because of the high sequence identity, each antibody recognized both receptors. To test the binding of PILR on neutrophils to CD99 on endothelial cells, neutrophils were allowed to interact with primary isolated WT or CD99−/− endothelial cells for 30 minutes prior to immunoprecipitation with anti-PILR antibodies. Coprecipitation of CD99 with PILR was only observed with WT, but not with CD99−/− endothelial cells (Figure 6D). Thus, PILR on neutrophils interacts with endothelial CD99. These results imply that CD99 on neutrophils could not be coprecipitated with neutrophil PILR. In line with this, we could not coprecipitate CD99 with PILR from neutrophil detergent extracts (not shown). This lack of interaction is likely a result of differences in glycosylation of CD99, as indicated by different apparent molecular weights of endothelial and neutrophil CD99 (Figure 6E).
Because the coprecipitation experiments required detergent lysis of the cells prior to antibody incubation, we wanted to test whether PILR on the cell surface of intact neutrophils is indeed accessible for CD99 in trans. To this end, a photoactive, transferable biotin label (supplemental Material and Methods) was conjugated to primary amines on CD99 Fc. This tag contained a second photoactive functional group, a biotin tag, and a cleavable disulfide spacer. These features allow the sequential crosslinking of interacting proteins (by photoactivation) and transference of the biotin affinity tag from 1 protein to its binding partner (by reduction of the disulfide spacer). We incubated neutrophils with CD99 Fc that was conjugated to this multifunctional label, induced crosslinking by photoactivation, and precipitated the products with streptavidin-coupled beads followed by immunoblotting for PILR. As shown in Figure 6F, we found that exogenous CD99 was able to directly transfer the tag to PILRs on neutrophils, whereas this was not seen with a control protein conjugated with this tag. In 3 experiments, we found that 1.2%, 4.0%, and 8.2% of all PILR molecules had received the biotin tag from CD99 Fc. Of note, PILR molecules interacting with CD99 differed slightly in their electrophoretic mobility from the majority of PILR molecules that were detectable in cell lysates (Figure 6F), suggesting that CD99 interacts in trans with a subset of PILR molecules that might differ in their posttranslational modifications.
Binding of neutrophils to CD99 modulates adhesion to ICAM-1
The hitherto unknown contribution of endothelial CD99 to neutrophil arrest in inflamed venules prompted us to analyze whether CD99 binding to neutrophils would influence β2-integrin–mediated binding to ICAM-1. To this end, we analyzed (in the presence of CXCL1) adhesion of mouse neutrophils under shear conditions to immobilized ICAM-1 Fc and P-selectin Fc and coimmobilized either CD99 Fc or human IgG (huIgG) as a control. To induce shear stress, we performed our adhesion assays in rotating Petri dishes. The highest number of bound cells was found near the center of the dish where shear was lowest, whereas the number of bound cells gradually decreased toward the perimeter of the dish where shear forces were higher. When the slope profiles of the decreasing numbers of bound neutrophils under increased shear were compared for immobilized CD99 Fc or huIgG, we found that the decrease in cell attachment under increasing shear was less pronounced in the presence of CD99 Fc. Calculating a shear resistance index from these measurements (supplemental Material and Methods) revealed that CD99 Fc enhanced shear resistance of neutrophil attachment to the P-selectin/ICAM-1–coated surface (Figure 7A). Importantly, this CD99-mediated support of adhesion under flow conditions was not observed in the absence of ICAM-1 Fc (Figure 7A). These results are in agreement with our intravital microscopy results. Collectively, our in vivo and in vitro results suggest that CD99 supports neutrophil binding to ICAM-1 under shear.
CD99 Fc supports shear resistance of the binding of neutrophils to ICAM-1 in a PILR-dependent manner. (A) Polymorphonuclear neutrophils (PMNs) were added together with CXCL1 to rotating Petri dishes (80 rpm) with immobilized ICAM-1 Fc, P-selectin (P-sel) Fc, and either CD99 Fc (blue bars) or huIgG (red bars) (as indicated) at room temperature for 30 minutes, followed by washing and fixation (left panel). PMNs attached at different distances from the center were counted by microscopy. The depicted shear resistance index represents the shear rate increase required for a set reduction of PMN binding (n = 10). Note that no increase was seen when ICAM-1 FC was omitted (n = 6) (right panel). (B) Role of PILRs for CD99-mediated shear resistance of PMN binding was investigated by repeating experiment described in panel A with PMNs pretreated with antibodies from preserum (left) (n = 16) or anti-PILR antibodies (right) (n = 9). (C) PMNs were passed through a flow chamber at 5 dyn/cm2 coated with P-selectin Fc, CXCL1, ICAM-1 Fc, and either CD99 Fc (blue bars) or huIgG (red bars) for 10 minutes at room temperature followed by enumeration of adherent cells (n = 15 [with ICAM-1 Fc] or n = 6 [without ICAM-1 Fc]). (D) Cells from bone marrow were passed through a flow chamber at 5 dyn/cm2 coated with P-selectin Fc, CXCL1, and either CD99 Fc (blue bars) or huIgG (red bars) for 5 minutes at room temperature followed by fluorescence-activated cell sorting analysis for binding of soluble ICAM-1 Fc. The ICAM-1-binding fraction of Gr-1+ cells incubated with the huIgG-coated surfaces was set as 1 (n = 6). n.s., *P < .05, and ***P < .001, as per 1-sample t test; #P < .05, as per unpaired t test; error bars show standard error of the mean.
CD99 Fc supports shear resistance of the binding of neutrophils to ICAM-1 in a PILR-dependent manner. (A) Polymorphonuclear neutrophils (PMNs) were added together with CXCL1 to rotating Petri dishes (80 rpm) with immobilized ICAM-1 Fc, P-selectin (P-sel) Fc, and either CD99 Fc (blue bars) or huIgG (red bars) (as indicated) at room temperature for 30 minutes, followed by washing and fixation (left panel). PMNs attached at different distances from the center were counted by microscopy. The depicted shear resistance index represents the shear rate increase required for a set reduction of PMN binding (n = 10). Note that no increase was seen when ICAM-1 FC was omitted (n = 6) (right panel). (B) Role of PILRs for CD99-mediated shear resistance of PMN binding was investigated by repeating experiment described in panel A with PMNs pretreated with antibodies from preserum (left) (n = 16) or anti-PILR antibodies (right) (n = 9). (C) PMNs were passed through a flow chamber at 5 dyn/cm2 coated with P-selectin Fc, CXCL1, ICAM-1 Fc, and either CD99 Fc (blue bars) or huIgG (red bars) for 10 minutes at room temperature followed by enumeration of adherent cells (n = 15 [with ICAM-1 Fc] or n = 6 [without ICAM-1 Fc]). (D) Cells from bone marrow were passed through a flow chamber at 5 dyn/cm2 coated with P-selectin Fc, CXCL1, and either CD99 Fc (blue bars) or huIgG (red bars) for 5 minutes at room temperature followed by fluorescence-activated cell sorting analysis for binding of soluble ICAM-1 Fc. The ICAM-1-binding fraction of Gr-1+ cells incubated with the huIgG-coated surfaces was set as 1 (n = 6). n.s., *P < .05, and ***P < .001, as per 1-sample t test; #P < .05, as per unpaired t test; error bars show standard error of the mean.
Antibodies against PILR, but not control antibodies, inhibited the supporting effect of CD99 on adhesion under flow to P-selectin/ICAM-1–coated surfaces (Figure 7B). We concluded that PILRs on the surface of neutrophils are involved in the CD99-induced support of neutrophil adhesion to ICAM-1 under flow.
In addition to the rotation-adhesion assays, we performed adhesion assays in flow chambers. Neutrophils were passed through flow chambers coated with P-selectin Fc, CXCL1, and ICAM-1 Fc, and either CD99 Fc or huIgG, followed by enumeration of adherent cells. More cells were bound onto the CD99-Fc–cocoated surface (Figure 7C). In addition, a similar assay was performed, except that ICAM-1-Fc cocoating was omitted, and binding to soluble ICAM-1 Fc was measured by fluorescence-activated cell sorting subsequent to the flow chamber treatment. The ICAM-1 binding fraction of neutrophils increased when they had contacted CD99 Fc (Figure 7D), further supporting our conclusion that CD99 interaction with neutrophils enhances β2-integrin activation.
Discussion
CD99 is well known to support leukocyte extravasation at the level of diapedesis through the vessel wall. Evidence thus far has been based on the use of anti-CD99 antibodies that interfere with the diapedesis process at the level of the interphase between endothelial cells and the underlying basement membrane.12-14,30 Here we report that CD99 gene inactivation in mice causes the same effect, demonstrating in an antibody-independent manner that CD99 is indeed required for the diapedesis process in vivo at a step beyond the luminal surface of endothelial cells. In addition, and unexpectedly, we found that CD99 also supports chemokine-induced arrest of leukocytes at the luminal surface of inflamed blood vessels. This newly discovered function as well as total leukocyte extravasation required CD99 only on endothelial cells, whereas CD99 on leukocytes was dispensable. On the basis of these results, we searched for heterophilic ligands and found that the PILRs on neutrophils bind to endothelial CD99. Blocking of this interaction with antibodies against PILRs abolished the enhancing effect of CD99 on shear resistance of neutrophil attachment to immobilized ICAM-1 and P-selectin. Collectively, these results suggest that the binding of endothelial CD99 to PILRs on neutrophils promotes neutrophil arrest under flow, which represents a novel mechanism whereby CD99 supports neutrophil extravasation.
Analyzing bone marrow–chimeric mice, we established that the contribution of CD99 for the extravasation of neutrophils depends exclusively on endothelial CD99, whereas CD99 on neutrophils was dispensable. This could be confirmed in transmigration assays with primary isolated endothelial cells and neutrophils selectively lacking CD99 (Figure 4). Our results are also in agreement with anti-CD99 antibody effects in in vitro transmigration assays with mouse neutrophils and endothelial cells.12 All of these results imply that mouse neutrophils express heterophilic ligands for endothelial CD99. In contrast, transmigration of mouse TH1 memory/effector T cells through cultured endothelial cells required CD99 on both cell types.11 Likewise, transmigration of human neutrophils and monocytes through cultured human endothelial cells required CD99 on the leukocyte and the endothelial side.31 This suggests that, dependent on leukocyte subtype and species origin, endothelial CD99 supports leukocyte transmigration by interacting with either heterophilic or homophilic binding partners.
A function of CD99 as a supporter of leukocyte arrest to the luminal surface of endothelial cells has not been shown before. Until now, the contribution of CD99 to leukocyte extravasation in vivo was only studied with function-blocking antibodies against CD99. These antibodies blocked leukocyte extravasation in various in vivo inflammation models and various tissues such as skin, peritoneum, and cremaster.11,12,14,30 However, neither intravital microscopy12 nor whole-mount stainings12,14 revealed a reduction in the number of neutrophils adhering to the vessel wall. Although the reason is presently unknown, our results emphasize the importance of analyzing the function of an adhesion receptor by gene inactivation, in addition to studying indirect effects caused by antibody-blocking experiments. The relevance of endothelial CD99 for intraluminal adhesion of leukocytes in vessels of the cytokine-inflamed cremaster was further specified by demonstrating that rapid leukocyte arrest, induced by intra-arterial administration of CXCL1, was strongly reduced by the absence of CD99. Given that chemokine-induced neutrophil arrest is based on the activation of the β2-integrin LFA-1, our results suggest that CD99 binding to neutrophils supports this process.
In agreement with this interpretation, we showed that the binding of neutrophils to immobilized ICAM-1 Fc and P-selectin Fc under flow, in the presence of CXCL1, improved if CD99 Fc was coimmobilized. Because no binding was seen when only P-selectin Fc and CD99 Fc were coated, this strongly suggests that CD99 improves the binding of neutrophils to ICAM-1. In line with this, blocking the PILRs on neutrophils with antibodies abolished the supportive effect of CD99, further suggesting that the CD99-PILR axis induces signals that support the CXCL1-induced process, which allows leukocytes to switch from rolling on P-selectin to firm adhesion to ICAM-1. It was reported that gene ablation of the inhibitory receptor PILR-α enhances adhesion of neutrophils to ICAM-1, and clustering of PILR-α triggered immunoreceptor tyrosine-based inhibitory motif–mediated signaling, which modulated β2-integrin inside-out activation.19 Thus, PILRs have the potential to modulate integrin activation.
Of note, we did not see changes in neutrophil rolling velocity in inflamed venules of CD99 gene–inactivated mice. Thus, CD99 supports β2-integrin–mediated adhesion required for stable arrest, but not the integrin contribution to leukocyte rolling. Because the function of intracellular binding partners as well as the activated integrin conformations differ for the 2 processes,32-34 it will be interesting to investigate in the future which of these processes is influenced by PILRs.
The only known heterophilic binding partners of CD99 are the PILRs. They have been described as lectins on NK cells and dendritic cells, which bind to O-linked carbohydrates of CD99 on lymphocytic cells.15 An interaction between endothelial CD99 and PILRs on neutrophils has not yet been demonstrated. Despite the lectin character of PILRs, which could principally allow them to bind to carbohydrates on various carrier glycoproteins,18 we found that the binding of endothelial cells to immobilized PILR-α and PILR-β was completely abolished by antibodies against CD99 or by blocking the expression of CD99 by small-interfering RNA. This establishes CD99 as the only endothelial cell surface ligand for the PILRs, which is detectable in this assay. This selectivity for 1 major ligand may suggest that not only carbohydrates, but also a part of the protein, may contribute to this receptor-ligand interaction.
PILR-α and PILR-β are both expressed by neutrophils.15 However, it was shown that a CD99-Fc fusion protein, when offered as a soluble reagent, did not bind to neutrophils, possibly as a result of the blocking of PILR by carbohydrate ligands in cis.19 Sialidase treatment of neutrophils enhanced the binding of CD99 Fc, which suggests that a significant fraction of PILR molecules is engaged in cis interactions with sialic acid–bearing cell surface molecules on neutrophils. We could confirm that soluble CD99 Fc, when incubated with neutrophils, did not give rise to significant signals detectable by fluorescence-activated cell sorting analysis (not shown). Despite this, we showed that immobilized CD99 Fc supported shear resistance of neutrophil adhesion to coimmobilized ICAM-1 Fc and P-selectin Fc. In addition, we demonstrated that endothelial CD99 could be coprecipitated with PILRs from neutrophils adhering to endothelial cells. Furthermore, we found that soluble CD99 Fc can directly, although weakly, interact with PILRs on the surface of neutrophils, as indicated by covalent tag transfer. We assume that multivalency of CD99 as it can be achieved by immobilization of CD99-Fc or as it may be provided by the presentation of CD99 on the endothelial cell surface might allow CD99 to bind to neutrophil PILR with higher avidity than soluble CD99-Fc.
In conclusion, we show here that CD99 supports 2 steps of the leukocyte extravasation cascade in vivo: the exit of neutrophils from the endothelial cell/ basement membrane interphase and the chemokine-induced arrest of neutrophils on the intraluminal vessel surface. CD99 on neutrophils is dispensable for these processes, suggesting a role for heterophilic ligands. Such ligands might be PILRs, given that we found that endothelial CD99 interacts with PILRs on neutrophils, and CD99 supports shear resistance of neutrophil adhesion to ICAM-1 in a PILR-dependent manner.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Lydia Sorokin for generously providing the antilaminin α5 monoclonal antibody 4G6.
This work was supported by funds from the Deutsche Forschungsgemeinschaft (SFB1009-A1) (D.V.) and from the Max Planck Society, and was performed as part of the Deutsche Forschungsgemeinschaft Clusters of Excellence Cells in Motion program.
Authorship
Contribution: D.G. performed, analyzed, and designed experiments and wrote the manuscript; S.M. and Y.-T.L. performed, analyzed, and designed experiments and wrote parts of the manuscript; A.A., R.S., K.S., M.P.-B., and D.J., performed, analyzed, and designed experiments; M.G.B., M.A., K.A., and K.-I.Y. provided valuable reagents; and D.V. initiated the study, designed the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dietmar Vestweber, Max Planck Institute of Molecular Biomedicine, Röntgenstr 20, D-48149 Münster, Germany; e-mail: vestweb@mpi-muenster.mpg.de.
References
Author notes
D.G. and S.M. are joint first authors.

![Figure 6. CD99 on EC represents the ligand for PILRs on neutrophils. (A) bEnd.5 cells were either untreated or pretreated with antibodies from preserum or anti-CD99 (as indicated) and subsequently allowed to adhere onto a surface immobilized with E-selectin Fc (control; red bar), PILR-α Fc (blue bars), or PILR-β Fc (light green bars) constructs for 60 minutes at 37°C (n > 17 from 3 independent experiments). (B) bEnd5 cells were pretreated either with control small-interfering RNA (siRNA) or with CD99 siRNA (as indicated), and adhesion assays were performed as described in panel A (n > 16 from 3 independent experiments). (C) Endothelial cells used in panel B were immunoblotted for CD99 or for tubulin (as indicated) after treatment with either control or CD99 siRNA. (D) Polymorphonuclear neutrophils (PMNs) were allowed to adhere to TNF-α–treated primary lung endothelial cells isolated from WT mice or from CD99 knockout mice (as indicated) for 30 minutes at 37°C. The cell mix was then subjected to immunoprecipitation with anti-PILR antibodies or control IgG (as indicated). Immunoprecipitates (left panel) or cell lysates (right panel) were immunoblotted for CD99 and PILR (as indicated). (E) Lysates of mouse primary endothelial cells and PMNs were immunoblotted for CD99. Note the difference in electrophoretic mobility. (F) CD99 Fc or huIgG were labeled with sulfo-N-hydroxysuccinimidyl-2-(6-[biotinamido]-2-[p-azido benzamido]-hexanoamido) ethyl-1,3′-dithioproprionate biotin and separately incubated with PMNs for 1 hour at 37°C, followed by photoactivation (312 nm ultraviolet light) to crosslink with the interacting binding partners for 10 minutes. Lysates were prepared from washed cells and crosslinked biotinylated proteins were immunoprecipitated (IP) by streptavidin and immunoblotted for PILR. Samples were reduced by 100 mM dithiothreitol to complete the biotin transfer. Data in panels D and F are representative of 2 (D) and 3 (F) independent experiments. *P < .05, ***P < .001. Error bars show standard error of the mean. One-way analysis of variance was followed by the Holm-Šídák test for panels A and B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/13/10.1182_blood-2016-08-733394/4/m_blood733394f6.jpeg?Expires=1767775515&Signature=DHlksmGGflQ-Z7OdbNyn4yF5ROkcyTfV3TW5wrMTfQ~lrgBLpEKE9IkcrAt7e85RIchqGbgiO9sMZpNnSzKWQYJpvLmzOzfdEyrw4tnSqOzlJK5qt94cQmaL9d7WzGj-awHsxNpuag3R-9c54Rcycg9ideZ-h6VZUnqbvCZn~3iuq6eueTDZpW0P3iJNkvDZv9gg21e5ec-Jq2k6Pvk7aqbiq9ss7jhFjJ1vEQYQ2jG3F7gt~TEYPHT95h0vpIe8vBV-5h9Oo5V0MwoR9jG~GNDmv5ZNn7h8QDg4eYO9HEm-Jy4LmZTN0DvuGm2JxuZleSfIZPkZNEZTDTqOv4~gsw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. CD99 Fc supports shear resistance of the binding of neutrophils to ICAM-1 in a PILR-dependent manner. (A) Polymorphonuclear neutrophils (PMNs) were added together with CXCL1 to rotating Petri dishes (80 rpm) with immobilized ICAM-1 Fc, P-selectin (P-sel) Fc, and either CD99 Fc (blue bars) or huIgG (red bars) (as indicated) at room temperature for 30 minutes, followed by washing and fixation (left panel). PMNs attached at different distances from the center were counted by microscopy. The depicted shear resistance index represents the shear rate increase required for a set reduction of PMN binding (n = 10). Note that no increase was seen when ICAM-1 FC was omitted (n = 6) (right panel). (B) Role of PILRs for CD99-mediated shear resistance of PMN binding was investigated by repeating experiment described in panel A with PMNs pretreated with antibodies from preserum (left) (n = 16) or anti-PILR antibodies (right) (n = 9). (C) PMNs were passed through a flow chamber at 5 dyn/cm2 coated with P-selectin Fc, CXCL1, ICAM-1 Fc, and either CD99 Fc (blue bars) or huIgG (red bars) for 10 minutes at room temperature followed by enumeration of adherent cells (n = 15 [with ICAM-1 Fc] or n = 6 [without ICAM-1 Fc]). (D) Cells from bone marrow were passed through a flow chamber at 5 dyn/cm2 coated with P-selectin Fc, CXCL1, and either CD99 Fc (blue bars) or huIgG (red bars) for 5 minutes at room temperature followed by fluorescence-activated cell sorting analysis for binding of soluble ICAM-1 Fc. The ICAM-1-binding fraction of Gr-1+ cells incubated with the huIgG-coated surfaces was set as 1 (n = 6). n.s., *P < .05, and ***P < .001, as per 1-sample t test; #P < .05, as per unpaired t test; error bars show standard error of the mean.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/13/10.1182_blood-2016-08-733394/4/m_blood733394f7.jpeg?Expires=1767775515&Signature=eU92zJcGgJJRdpeUZ51EkvxTy-i8v8vLSIjSWApI7EEDHC828FbMBq7n11Gg1u8kTMsXFe0oKWf8sg574njxKBVR0p4mdwNJIK90hbAlJh1xdgLazXAM4SsZXOLkmRKGD26irQHUjmDWMXYV9a8cyB2LpSH2FNZnwCZ88KwBdWh9pQsSGjieVfIWblOyUpVCUWBOktL6mRjXKU2q7hChjldWN4gWSqSUBTVfIpwIIlnewImS8Dmn1c9nt4iDqopj3hY-TgwJZ5GVxuD6i6ZntNiXDF5HnU4wKoIf8yqWafh44nrFHhqSk8WAlOg38gszkhRnkoKoO5BLtYnxhhGvPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal