In this issue of Blood, Verhoef et al provide evidence that platelets contain polyphosphate polymers of sufficient size to promote activation of factor XII (FXII), thereby addressing a long-standing enigma as to the potential contribution of platelet polyphosphate to thrombosis.1
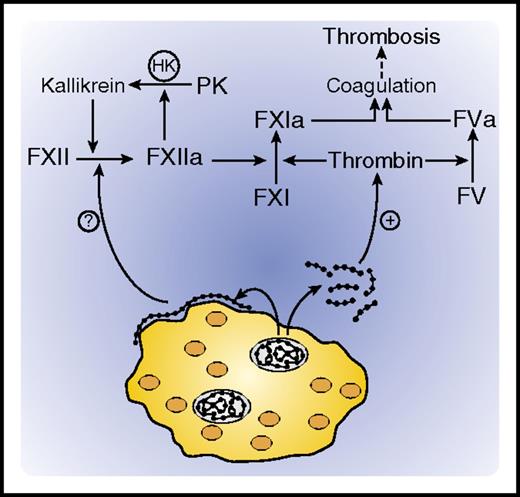
Activation of coagulation by platelet polyphosphate. Platelet dense granules contain polyphosphate polymers (shown as beads) and high concentrations of calcium. Upon platelet activation, polyphosphate polymers of varying sizes are released. Shorter polymers are soluble and promote coagulation by serving as cofactors for thrombin-mediated activation of FXI and FV, whereas longer polymers deposit as nanoparticles on the platelet surface, where they may initiate coagulation by activating FXII. HK, high molecular weight kininogen; PK, prekallikrein.
Activation of coagulation by platelet polyphosphate. Platelet dense granules contain polyphosphate polymers (shown as beads) and high concentrations of calcium. Upon platelet activation, polyphosphate polymers of varying sizes are released. Shorter polymers are soluble and promote coagulation by serving as cofactors for thrombin-mediated activation of FXI and FV, whereas longer polymers deposit as nanoparticles on the platelet surface, where they may initiate coagulation by activating FXII. HK, high molecular weight kininogen; PK, prekallikrein.
Platelet dense granules contain inorganic polyphosphate (polyP) and divalent metal ions, which are released upon platelet activation. PolyP consists of varying-length chains of negatively charged phosphate units. A potent procoagulant, polyP initiates coagulation by binding FXII and promoting its autoactivation. The conundrum is that polyP secreted by platelets consists of 60 to 100 phosphate units and is a less efficient activator of FXII than are polyP polymers consisting of 200 or more phosphate units, such as those found in microorganisms. How then does platelet polyP activate FXII? Verhoef and colleagues provide a potential answer. They show that the polyP released from platelet-dense granules aggregates into nanoparticles that accumulate on the platelet surface and are of sufficient size to promote FXII activation.
PolyP is ubiquitous in nature and is found in eukaryotic and prokaryotic organisms where it modulates such diverse functions as energy metabolism, metal ion sequestration, and cell viability.2 Interest in platelet polyP started with its detection in dense granules, but elucidation of its procoagulant activity came later. Thus, it was shown that platelet-derived polyP triggers FXII activation in vivo, because its injection induced fatal pulmonary embolism and increased vascular permeability in wild-type mice, but not in FXII-deficient mice.3 Although these findings have been questioned, if correct, they provide a plausible explanation for the bleeding diathesis that occurs in patients with Hermansky-Pudlak syndrome, whose platelets are deficient in dense granules and contain lower concentrations of polyP than do normal platelets. Despite these observations, however, prior to the work of Verhoef and colleagues, it remained unclear how platelet polyP could activate FXII.
While investigating a probe for detecting platelet-bound polyP, this group previously observed that exogenous long-chain polyP binds to platelets, whereas short-chain polyP does not.4 They also showed accumulation of endogenous polyP on the surface of activated platelets. In the current study, Verhoef and colleagues demonstrate that, whereas the total polyP in platelet lysates promotes contact activation in a FXII-dependent manner, the soluble polyP fraction does not. These results suggest that platelets contain long-chain polyP, but do not necessarily release it when they are activated. Using confocal and electron microscopy, Verhoef et al visualized spherical polyP nanoparticles ranging in diameter from 100 to 200 nm on the granulomere of spread platelets. Formation of these nanoparticles appears to be dependent on polyP precipitation by divalent metal ions, because exposure of platelets to ethylenediaminetetraacetic acid (EDTA) abrogated their accumulation. Furthermore, Ca2+ addition to short-chain polyP increased its apparent polymer size and promoted its capacity to activate FXII, whereas addition of EDTA attenuated these phenomena. Therefore, Ca2+ appears to modulate the structure and properties of polyP.
Although this study identifies new avenues for investigation of platelet-derived polyP, questions remain. Direct evidence is lacking that the polyP on the platelet surface consists of longer chains that promote contact activation. Instead, this premise was inferred from studies using fractionated platelet lysates and synthetic polyP polymers of varying lengths. Additional work is needed to directly quantify the size and procoagulant activity of the polyP on the platelet surface, because if the membrane-bound polyP is partially resistant to degradation by phosphatase, as the current study shows, it may not be sufficiently accessible to promote coagulation. Therefore, even though it is now clear that polyP nanoparticles accumulate on the surface of spread platelets, it remains uncertain whether this population of platelet polyP is the major driver of coagulation.
Platelets release polyP and Ca2+ when they are activated. Although the released polyP is a poor activator of FXII, it still can enhance thrombin generation by promoting thrombin-mediated activation of FXI and FV (see figure). In addition, if Ca2+ modulates the physical state of polyP, it is possible that the high concentration of Ca2+ in the dense granules maintains the sequestered polyP in a more stimulatory nanoparticle form. When the local levels of Ca2+ fall because of the dilution that occurs with dense granule release, it is also possible that the nanoparticles will progressively lose their FXII-dependent stimulatory activity. Therefore, platelets may contain and release polyP chains of varying lengths depending on the local Ca2+ concentration, with only the longest polymers remaining associated with the platelet surface.
The study by Verhoef et al raises new questions about the interaction of polyP with the platelet membrane. Recent work has shown that, upon platelet activation, certain proteins assemble on their surface in polar caps. These bound proteins include plasminogen, fibrinogen, FXII, FIX, FX, FVa, FVIIIa, and prothrombin.5,6 Remarkably, polyP binds many of these proteins, thereby raising the possibility that the polyP nanoparticles that deposit on the surface of activated platelets provide the nidus onto which these proteins assemble. If this is the case, then what anchors polyP to the platelet membrane? Additional studies are needed to address this question.
In conclusion, the study of Verhoef et al provides further support for the concept that platelet polyP is an important driver of coagulation. The physiological significance of polyP is demonstrated by the ability of polyP-neutralizing agents to attenuate thrombosis in animal models.7,8 Therefore, in addition to the race to develop inhibitors of FXI and FXII as anticoagulants, neutralizing agents against polyP may provide another approach to attenuating thrombosis without disrupting hemostasis.9
Conflict-of-interest disclosure: The authors declare no competing financial interests.