Abstract
Acquired severe aplastic anemia (SAA) is a rare hematologic disease associated with significant morbidity and mortality. Immune destruction of hemopoietic stem cells plays an important role in pathogenesis, as shown by successful treatment with immunosuppressive agents, leading to transfusion independence or complete recovery of peripheral blood counts in a proportion of patients. Growth factors can be combined with immunosuppressive therapy (IST) and may improve response rates, as recently shown with thrombopoietin analogs. Anabolic steroids may still play a role in combination with IST. The problem with IST is failure to respond and the development of late clonal disorders. Bone marrow transplantation (BMT) is the other therapeutic option: a matched sibling donor remains the best choice. For patients lacking a matched family donor, unrelated donors can be readily found, although mostly for patients of Caucasian origin. Other BMT options include unrelated cord blood or mismatched family donors. Acute and chronic graft-versus-host disease remain important complications of BMT. Patient age is a strong predictor of outcome for both IST and BMT, and must be considered when designing therapeutic strategies. Early diagnosis and treatment, as well as long-term monitoring, remain crucial steps for successful treatment of SAA.
Clinical presentation
Pathogenesis
Acquired SAA is regarded as the result of an immune-mediated destruction of hematopoietic cells, at least in a proportion of patients.1 The emergence of late clonal disorders in 10% to 20% of patients after immunosuppressive therapy (IST)2 raises the questions of whether some patients with SAA actually have a premalignant disease and whether IST is just postponing the inevitable.3 Support for this view has come from the identification of somatic mutations involving telomerase RNA component (TERC) and telomerase reverse transcriptase (TERT)4 and, more recently, involving myeloid cancer candidate genes in a significant proportion of patients.5-7
Diagnosis and early intervention
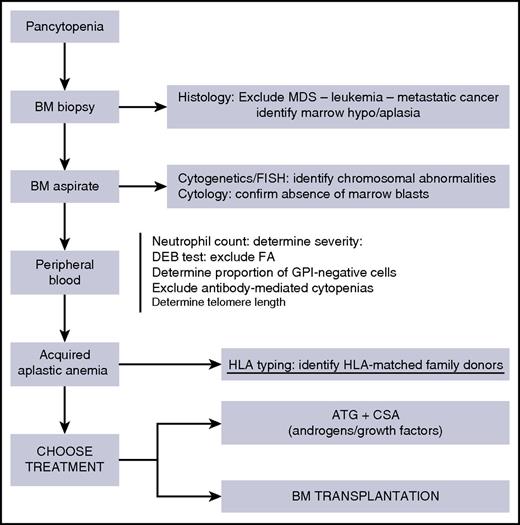
The diagnosis of acquired SAA is based on the exclusion of other disorders that can cause pancytopenia and on the well-known Camitta criteria8 (Figure 1). A BM biopsy is mandatory (preceded by platelet transfusions if the platelet count is below 20 × 109/L) and will confirm an empty marrow; it should also exclude a MDS or leukemia, as well as marrow metastasis from solid tumors (Figure 1). A BM aspirate will be used for cytogenetics and/or FISH analysis to determine chromosomal abnormalities. Whether the identification of chromosomal abnormalities is compatible with the diagnosis of SAA is debated9 ; clearly, some abnormalities carry a poor prognosis (such as deletion of chromosome 7), whereas others (such as +Y and +8) are more benign and may not affect the therapeutic strategy.9 Identification of a paroxysmal nocturnal hemoglobinuria (PNH) clone by flow cytometry will help exclude an inherited form of marrow failure and may suggest this is not a hypoplastic MDS (Figure 1); a negative diepoxybutane test will exclude FA. Determination of telomere length is not mandatory, but will help exclude telomeropathies.4
Diagnostic procedures in patients with pancytopenia. ATG, antithymocyte globulin; BM, bone marrow; CsA, cyclosporine A; DEB, diepoxybutane; FA, Fanconi’s anemia; FISH, fluorescent in situ hybridization; GPI, glycosyl phosphatidyl inositol; MDS, myelodysplastic syndrome.
Diagnostic procedures in patients with pancytopenia. ATG, antithymocyte globulin; BM, bone marrow; CsA, cyclosporine A; DEB, diepoxybutane; FA, Fanconi’s anemia; FISH, fluorescent in situ hybridization; GPI, glycosyl phosphatidyl inositol; MDS, myelodysplastic syndrome.
Severity can be determined by neutrophil counts: patients with 0 to 0.2, 0.21 to 0.5, and >0.5 polymorphonuclear cells (PMNs) × 109/L are classified, respectively, as very severe, severe, and nonsevere aplastic anemia,10 and severity has been a strong predictor of survival in patients receiving IST.10,11 Patients with SAA require early diagnosis and intervention, whether IST or BMT, because the interval between diagnosis and treatment is another strong predictor of survival.12 Transfusion policies are important in the early days of diagnosis, and guidelines for supportive care have been published.13 In approximately 5% of patients, SAA will follow an episode of elevated transaminase and hyperbilirubinemia,14 although the search for hepatitis A, B, and C virus (HAV, HBV, and HCV) is typically negative, as well as the search for other viruses. Abnormal liver function test and/or elevated bilirubin levels should not stop therapeutic strategies.
Initially, one should concentrate on the diagnosis,15 with some key tests, as outlined in Figure 1. The BM biopsy is the diagnostic procedure with the highest level of accuracy. In the meantime, the patient will be transfused according to guidelines.13 Once the diagnosis has been ascertained, HLA typing of the patient and his/her family should be one of the first interventions in a patient with SAA, certainly in patients younger than 60 years of age.
HLA identical sibling transplantation
BMT or IST
If an HLA-matched family donor is identified, marrow transplantation should be the first-line therapy in patients younger than 40 years (Figure 2); this is based on studies comparing HLA-identical BMT vs first-line IST.11,16 However, the advantage in failure-free survival for a young patient with a low neutrophil count declines with increasing age11 as a result of higher mortality after HLA-identical BMT in patients aged 21 to 40 years or older than 40 years.
Treatment strategy in patients with acquired aplastic anemia. Patients are stratified according to whether or not they have an HLA-identical sibling. In young patients (<40 years) with a matched donor, allogeneic BMT is first-line therapy. In patients older than 40 years, and for patients without a matched sibling, ATG+CsA should be first-line therapy. In selected children with very severe disease, an unrelated donor (UD) graft may be considered first-line therapy (dashed arrow). In patients aged 0 to 60 years, nonresponders (no resp) to ATG have several choices, depending on the performance status of the patient, the availability of an HLA-matched UD, and patient age. The options are an UD BMT, a second course of IST (ATG+CsA), or an alternative donor transplant (Alt Donor Tx, indicating haploidentical transplants or cord blood [CB] transplants). At older than 60 years of age, medical treatment is preferable over BMT. d+120 = day +120; EPAG, eltrombopag; HLA = Sib, HLA-identical sibling; Sib BMT, identical sibling transplantation; UD BMT, unrelated donor BMT.
Treatment strategy in patients with acquired aplastic anemia. Patients are stratified according to whether or not they have an HLA-identical sibling. In young patients (<40 years) with a matched donor, allogeneic BMT is first-line therapy. In patients older than 40 years, and for patients without a matched sibling, ATG+CsA should be first-line therapy. In selected children with very severe disease, an unrelated donor (UD) graft may be considered first-line therapy (dashed arrow). In patients aged 0 to 60 years, nonresponders (no resp) to ATG have several choices, depending on the performance status of the patient, the availability of an HLA-matched UD, and patient age. The options are an UD BMT, a second course of IST (ATG+CsA), or an alternative donor transplant (Alt Donor Tx, indicating haploidentical transplants or cord blood [CB] transplants). At older than 60 years of age, medical treatment is preferable over BMT. d+120 = day +120; EPAG, eltrombopag; HLA = Sib, HLA-identical sibling; Sib BMT, identical sibling transplantation; UD BMT, unrelated donor BMT.
The age effect
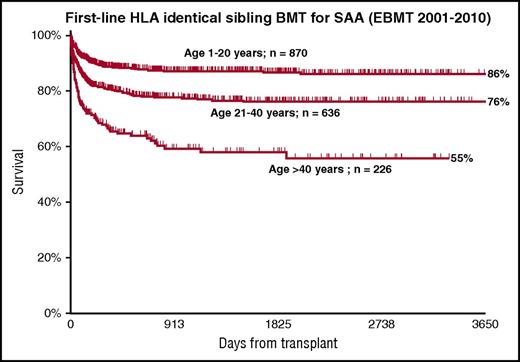
In patients grafted from matched siblings, there is a very strong age effect, with survival of 82%, 72%, and 53% for patients aged 1 to 20, 21 to 40, and older than 40 years,17 as a result of a higher incidence of graft failure and graft-versus-host disease (GVHD).17 European Group for Blood and Marrow Transplantation (EBMT) data for patients grafted from matched siblings in the decade from 2001 to 2010 show the same age effect (Figure 3): 86%, 76%, and 55% survival at 10 years. For this reason, IST should be first-line therapy in older patients, as shown in Figure 2, and BMT should be carefully considered for selected cases with good performance status and severe disease (Figure 2, dashed arrow).
A strong age effect in patients with aplastic anemia, after transplantation from an HLA identical sibling. Data from the EBMT registry.
A strong age effect in patients with aplastic anemia, after transplantation from an HLA identical sibling. Data from the EBMT registry.
The conditioning regimen
The standard conditioning regimen for matched sibling transplants is cyclophosphamide 200 mg/kg (CY 200) and ATG, as originally described.18 Although a randomized study failed to show an advantage for ATG,19 the same survival difference proved significant in a larger retrospective study20 This regimen is excellent for young patients, but CY 200 may be too toxic in older patients, although attempts have been made to reduce toxicity with fludarabine (FLU)-based regimens and lower doses of CY.21-24 Recent EBMT data (A.B., EBMT database, unpublished data) suggest that survival of patients older than 40 years can be significantly improved with a FLU-based regimen, in addition to ATG or alemtuzumab (CAMPATH), and is comparable to survival of patients in the 21- to 40-year-old age group (74% vs 75%). Current guidelines from EBMT25 and the British Society for Standards in Haematolgy26 call for a combination of FLU-CY with ATG (FCA) or alemtuzumab (CAMPATH) (FCC) for patients with SAA who are older than 30 years and receiving a matched sibling donor transplant. The CY dose to be combined with FLU is a matter of discussion, ranging from 40 to 120 mg/kg.
BM or peripheral blood
Two registry-based studies have shown that BM results in superior outcome as compared with peripheral blood (PB) in matched sibling transplants20,27 because of less acute and chronic GVHD with BM and comparable risk for rejection (2.5% for PB and 1.5% for BM). The recent British guidelines call for BM as a stem cell source in ATG-based conditioning.26 The evidence we currently have suggests BM should be the only acceptable stem cell source for transplants from HLA-identical siblings in SAA.
Alternative donor transplantation
Case report
A 23-year-old woman was referred to us in December 2011, having failed 2 courses of ATG and CsA. The patient had developed an invasive fungal infection after the IST, with lung lesions and a left pneumothorax. She was admitted on December 20, 2010, with drainage in her left pleural cavity, high temperature, and absolute pancytopenia. In addition, the patient had developed polyneuritis with almost complete tetraplegia. Her performance status was extremely poor. She received a conditioning regimen including FLU 120 mg/m2, CY 120 mg/kg, ATG (thymoglobulin; Sanofi France) 3.75 mg/kg2, and a BMT from an 8/8 mated UD. Prophylaxis of GVHD consisted of CsA and short-course methotrexate; rituximab 200 mg was given on day +5 to prevent Epstein-Barr virus (EBV) reactivation. Treatment with voriconazole was continued. Engraftment was rapid and complete, and the pleural drainage was removed 1 month later. There was no GVHD and no EBV reactivation, but neurologic rehabilitation was slow, and the patient remained admitted in the transplant unit for several months. Antifungal treatment was continued, and lyposomial amphotericin (AmBisome) was also introduced. Two years later, the patient underwent upper left lobectomy to remove her aspergillum lesions because of their proximity to large vessels. The patients is alive and well and off immunosuppression 4 and a half years posttransplant.
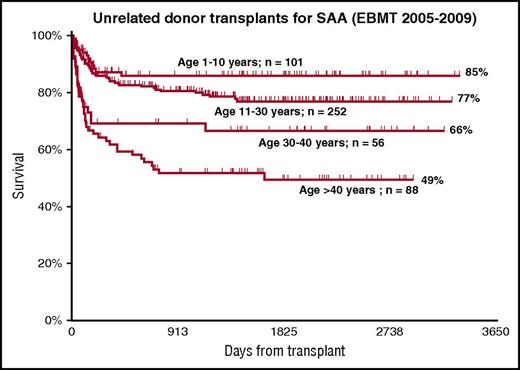
This case exemplifies the infectious complications of prolonged neutropenia and immune suppression in aplastic anemia, as well as showing that an UD BMT can be performed successfully, even in very sick patients with marrow failure, in keeping with the reported improved outcome of UD grafts.28,29 This case also suggests that in the presence of a matched UD, perhaps second-line therapy should be a UD transplant, rather than a second course of ATG (Figure 2). In the absence of a matched sibling donor, I usually start a search for an UD as early as possible, certainly when the patient is younger than 50 years of age. Early identification of an 8/8 HLA-A, -B, -C, or -DRB1–matched donor is always important, especially for pediatric patients, for whom up-front UD has shown to have 96% long-term survival, which is comparable to up-front sibling BMT.28 For this reason, Figure 2 outlines a possible dashed line for patients younger than 20 years, with an option to proceed to an UD BMT as first-line therapy, although a first course of IST remains standard of care, as suggested by identical survival at 2 years for first-line UD BMT and IST.28 UD transplantation should also be considered in patients failing a first course of IST, as already mentioned (Figure 2). Increasing patient age and HLA matching remain significant predictors of survival after UD grafts29-32 (Figure 4). I consider an 8/8 HLA-matched UD the optimal choice, possibly matched with the recipient, for cytomegalovirus sero-status.33
The age effect in UD transplants: best outcome is seen for very young patients, for whom first-line UD BMT may be considered. Data from the EBMT registry.
The age effect in UD transplants: best outcome is seen for very young patients, for whom first-line UD BMT may be considered. Data from the EBMT registry.
UDs and stem cell source.
In addition, for UD grafts, BM provides a survival advantage over PB; this has been shown in studies from EBMT and from the Center for International Blood and Marrow Transplant Research.34,35 In the recent EBMT analysis, PB was the strongest negative predictor of survival in multivariate analysis.34 The British guidelines26 suggest PB can be used as a stem cell source with CAMPATH as GVHD prophylaxis, despite significant inferior survival of PB vs BM transplants (P = .03) in the British report on patients grafted with the FCC conditioning regimen.36 I personally think the data strongly suggest we should always use BM as a stem cell source; an exception could be a second transplant because of graft rejection.
UD BMT and conditioning regimens.
Several studies have explored the combination of low-dose total body irradiation (TBI) with FLU-CY29,32,37-39 ; the dosage of both TBI and cyclophosphamide has been the topic of prospective studies.40,41 The current recommendation is CY at a dose between 50 and 100 mg/kg (total dose), TBI between 2 and 4 Gy, and FLU 100–150 mg/m2.26 Registry data (EBMT) for UD transplants show survival of 85%, 77%, 66%, and 49% for patients aged 1 to 10, 11 to 30, 31 to 40, and older than 40 years (Figure 4). The donor’s age appears to be an additional prognostic factor.42
The ideal UD is a male, HLA-matched donor at the A,B,C,DRB1 loci, younger than 30 years, cytomegalovirus matched with the recipient; the conditioning regimen would include FCA and low-dose TBI or FCC.
Unrelated cord blood transplants.
Unrelated CB transplants have been reported in 71 patients who received a single or double unrelated CB transplant,43 with 45% survival for patients receiving a total nucleated cell dose greater than 3.9 × 107/kg. The conditioning regimen for CB transplants is identical to the regimen proposed for UD transplants, although methotrexate is omitted from GVHD prophylaxis of CB grafts; 1 dose rituximab 150 mg is recommended on day +5 to prevent EBV lymphoproliferative disorders.43 Patients who do not have a suitable UD could be eligible for a CB graft if the nucleated cell dose is adequate (Figure 2).
Haploidentical transplants.
Young patients who do not have a matched UD or a suitable CB unit and who have failed at least 1 course of IST or have rejected a previous UD or CB graft are eligible for a transplant from HLA haploidentical family donors (HAPLO). Small series of patients have been reported with different conditioning regimens, many using high-dose posttransplant CY.44-49 A total of 84 patients were reported in these studies, and the average 1-year survival was 74%, which is very encouraging. Nevertheless, HAPLO grafts are still in the experimental stage and should be considered only after having failed at least 1 course of IST in the absence of a suitable UD (Figure 2, identified as Alt Donor Tx).
EBV lymphoproliferative disorders.
Patients with SAA undergoing alternative donor transplants, especially with an ATG-based regimen, are at high risk of developing EBV-related lymphoproliferative disorders. We have been using a small dose of rituximab (200 mg, fixed dose) on day +5 after transplantation50 for all patients receiving an alternative donor graft and ATG in the conditioning regimen, and this has eliminated the issue of EBV-related lymphoproliferative disorders.50
Rejection and autologous recovery.
Rejection is seen in 5% to 15% of patients with SAA undergoing a BMT and calls for immediate action with the attempt of rescuing the patient with a second transplant. The same donor can be used, usually collecting granulocyte colony-stimulating factor (G-CSF)-mobilized PB cells, but haploidentical grafts have also been shown to be effective in a proportion of patients.44-49 Measures to completely prevent rejection in SAA have not been identified: however, the risk will be reduced with the use of ATG, low-dose radiation, and FLU for UD grafts and a marrow cell dose greater than 2 × 108/kg. Some patients rejecting their graft can undergo autologous hematologic recovery51 ; this is a rare event, with an overall rate of 4.2%, but when it occurs, survival is excellent (84%).
Nontransplant therapy
ATG+CsA
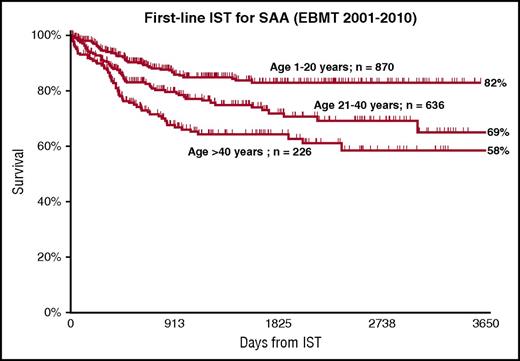
The standard regimen for first-line IST remains ATG and CsA, with hematological recovery in 50% to 70% of cases and excellent long-term survival among responders.15,52-58 First-line therapy is recommended for all patients without a matched sibling donor (Figure 2), but also for patients with a matched sibling who are older than 40 years.26 There is a strong age effect also after IST, as shown in Figure 5, with survival of 82% and 58% for patients younger than 20 or older than 40 years (A.B., EBMT database, unpublished data); this figure outlines survival after first-line IST and includes salvage therapy with a stem cell transplant for nonresponders. Failure-free or transplant-free survival would be clearly inferior16 and would favor transplantation, but only in younger patients and only when compared with transplants from matched sibling donors.11 For this reason, current guidelines recommend IST first-line therapy for all patients without a matched sibling donor.26 After failing a first course on IST, patients with a matched UD should undergo a transplant if clinically fit; the choice of a transplant vs a second course of IST will need to be patient-oriented.
The age effect in patients receiving first-line IST. Data from the EBMT registry.
The age effect in patients receiving first-line IST. Data from the EBMT registry.
Duration of CsA therapy
CsA should be given for a minimum of 6 months, but I tend to prolong CsA for more than a year, although at low doses. Once a response has been obtained, discontinuation should be very, very slow, with frequent evaluation of peripheral blood counts to identify early signs of relapse.59 I tend to reduce CsA until I give it 2 to 3 times/week, rather than daily: We are treating an autoimmune disorder, and these patients are really never cured of the disease.
Source of ATG
Horse ATG (ATGAM; Pfizer) has been compared with rabbit ATG (rATG) (Thymoglobulin; Sanofi France) in a prospective randomized trial and has been shown to be superior in terms of response at 6 months (68% vs 37%) and 3-year survival (96% vs 76%).60 Similar results have been obtained in a matched-pair analysis of the EBMT61 and in a retrospective pediatric Japanese study.62 One hypothesis to explain this difference has been a stronger immunosuppressive effect of rATG, leading to more early infections in patients receiving this product.60-62 Other studies, although not prospective, have failed to show significant differences between horse ATG and rATG.57,63,64 A large study on more than 800 patients receiving first-line therapy with rATG (Thymoglobulin) is currently being completed.
G-CSF
The combination of ATG+CsA with G-CSF has shown very good response rates and survival.65 However, 3 randomized studies comparing ATG+CsA with or without G-CSF have failed to show a survival advantage, for patients receiving G-CSF.66-68 In the most recent study,68 the neutrophil count on day +30 predicted response and survival in G-CSF patients. This is in keeping with our initial study,65 suggesting ATG+CsA+ G-CSF identifies early nonresponders, and perhaps may indicate an urgent transplant approach. The 3 randomized studies also confirmed that there was no increased incidence of malignant clonal disorders for G-CSF patients, disproving the reported increased risk for patients receiving prolonged G-CSF treatment shown in retrospective studies.69 I personally believe ATG+CsA+G-CSF remains an interesting first-line therapy because it allows for a rapid neutrophil recovery and identifies early nonresponders. There is no place for G-CSF as single-drug therapy.
Eltrombopag
The new player in the field of nontransplant therapy for SAA is EPAG: After initial encouraging results in refractory patients,70 EPAG has been used together with ATG +CsA as initial treatment in 88 patients.71 The overall response at 6 months was 85%, with survival on the order of 90%. Cytogenetic abnormalities and clonal evolution to myelodysplasia occurred at a similar frequency compared with standard IST, although follow-up is still short.71 The EBMT Working Party for Severe Aplastic Anemia is running 2 prospective randomized studies: 1 compares ATG+CsA with or without EPAG in SAA, and the other compares CsA with or without EPAG in non-SAA.
Case report
A 62-year-old man presented in October 2012 with severe pancytopenia (hemoglobin, 8.3 g/dL after transfusions; white blood cells, 0.8 × 109/L; PMNs, 0.3 × 109/L; platelets, 8 × 109/L), which had gradually developed over the course of a year.
The patient’s medical history revealed a liver transplant at the age of 50 for HCV+ HBV+ cirrhosis, and treatment with CsA since then. In 2011, with declining peripheral blood counts, a BM aspirate showed an empty marrow with trisomy 8 and −Y. The patient was diagnosed with aplastic anemia and received a first course of horse ATG in December 2011. Because of a lack of response, the patient received a second course of ATG (rATG) in July 2012. Four months later, a BM aspirate was unsuccessful, and a marrow biopsy showed complete aplasia. The patient continued to be cytopenic and dependent on red blood cells and platelet transfusions. An unrelated transplant was ruled out because of age and the lack of an 8/8 matched donor. The patient was continued on CsA; testosterone 40 mg by mouth was added 3 times a week, together with EPAG 50 mg every other day. The dose was kept low because of potential liver toxicity, and G-CSF was also given 3 times a week. PB counts improved gradually, and the patient was transfusion-independent after 3 months. In 2013, his counts were as follows: hemoglobin, 11.3 g/dL; white blood cells, 4.8 × 1099/L; PMNs, 3.7 × 109/L; platelets, 71 × 109/L. The patient was started on sofosbuvir and simeprevir for his HCV, and CsA was changed to tacrolimus. He was able to complete his anti-HCV therapy and is now 5 years post-ATG, HCV-negative, receiving low-dose tacrolimus, EPAG 25 mg thrice weekly, low-dose testosterone, and deferoxamine for iron overload, with ferritin reduced from 13.000 to 409 ng/mL. He has developed a small PNH clone (16% CD14, 3% CD16, 0.4% CD59).
Androgens
This patient is an example of an older adult with severe marrow failure, cytogenetic abnormalities, and comorbidities in whom combined treatment with growth factors, CsA, and androgens was successful. I often use this combination in frail patients. Androgens have shown encouraging results both in the animal model72 and in patients with marrow failure.73 A first randomized trial comparing ATG with or without androgens failed to show improved response and survival in the ATG+androgens group.74 However, in a second randomized trial, women receiving ATG+androgens had significantly superior response rates, although comparable survival, as with ATG without androgens.75 Intensified IST with ATG and androgens produced a 77% response rate and 78% survival at 5 years in a single-center study.76 We have shown a 59% response rate with testosterone 40 mg /day (5 days/week) in patients with persistent cytopenia after IST.77
Recently, sex hormones have been shown to induce telomere elongation in vitro,78 and also in vivo.79 Taken altogether, these studies point to a significant effect of androgens in patients with marrow failure, including increased telomerase activity. Perhaps we should reconsider their role in nontransplant strategies with ATG, CsA, and EPAG.
High-dose cyclophosphamide
The Baltimore group has reported encouraging results with the use of high-dose CY instead of ATG+CsA in 67 patients, with a response rate of 77% and a 10-year survival of 88%80 ; nevertheless, a prospective study has failed to show an advantage of CY over ATG and has highlighted an increased risk for infections.81 For these reasons, high-dose CY cannot be recommended as a nontransplant therapy outside a clinical trial.
Problems with IST
Patients treated with IST are not cured of their disease and are at risk for 3 major complications: no response, relapse, and clonal evolution. Patients not responding to a first course of ATG should be considered for an UD transplant if younger than 40 years (Figure 2); above the age of 40 years, a transplant from an unrelated or alternative donor is one possibility, but a second course of IST, or the addition of EPAG, are valid alternative options (Figure 2), and the choice should be made on the basis of the age of the patients, the clinical conditions, and the severity of the disease. Relapse is the second problem: it occurs in approximately 30% of responders, but can be successfully treated in the majority of patients with a second course of ATG or transplantation if a suitable donor is available. Clonal evolution is the third problem1-3 : the emergence, or existence from diagnosis, of a small GPI negative clone, as determined by flow cytometry, may be involved in the pathogenesis82 and may be a favorable prognostic indicator of response to IST83,84 ; a large GPI negative erythrocyte clone may lead to hemolysis and clinical PNH.85 The most worrisome problem is cytogenetic evolution or a myelodysplastic syndrome, which has been reported to occur in 18% of patients86 and calls for a transplant strategy for eligible patents.87 For this reason, morphologic and cytogenetic examination of marrow cells should be performed in patients with SAA after immunosuppressive treatment, possibly at yearly intervals.15,26
Treatment of patient older than 60 years
Transplantation is rarely offered to patients older than 60 years because of high transplant-related mortality. Patients between 60 and 70 years of age should be treated with conventional ATG+CsA, and I also use G-CSF because of faster neutrophil recovery. In a study looking at elderly patients, the EBMT has shown that response to IST also can be achieved in older patients.88 Above the age of 70 or 80 years, patients can be rather frail, and I often use CsA plus androgens and reserve CsA+ATG for fit patients, as suggested also by Scheinberg and Young15 .
Conclusions
Acquired aplastic anemia remains a difficult disease, with problems of diagnosis and treatment, and patients should be treated, preferentially, in experienced centers, and best in the context of clinical trials. Significant progress has been made in understanding the pathogenesis of the disease, and new treatment options are now available. Improved outcome of UD transplants has produced survival comparable to that of matched sibling donor grafts, although with increased risk for GVHD. Haploidentical transplants should be considered experimental, and are being used in severe cases lacking a suitable matched donor or CB unit; early results seem promising. EPAG could become a relevant actor in the field of nontransplant therapy and may change our treatment strategies if response and survival rates, recently reported, are confirmed. I often use androgens in patients who fail to achieve hematopoietic recovery after IST, and have seen a significant number of responses. In the seventies, the mortality of acquired SAA was on the order of 80% to 90%: we are currently looking at 80% to 90% survival, which is a fantastic result of worldwide research in this field.
Acknowledgment
This work was supported in part by Fondazione Ricerca Trapianto Midollo Osseo, Genoa, Italy.
Authorship
Contribution: A.B. wrote this review.
Conflict-of-interest disclosure: A.B. reports being on the speakers bureau for Sanofi, Pierre Fabre, Therakos, Miltenyi, ADIENNE, Gilead, and Novartis.
Correspondence: Andrea Bacigalupo, Istituto di Ematologia, Università Cattolica del Sacro Cuore, Fondazione Policlinico Universitario Gemelli, Largo Agostino Gemelli 1, Rome, Italy; e-mail: andrea.bacigalupo@unicatt.it.

![Figure 2. Treatment strategy in patients with acquired aplastic anemia. Patients are stratified according to whether or not they have an HLA-identical sibling. In young patients (<40 years) with a matched donor, allogeneic BMT is first-line therapy. In patients older than 40 years, and for patients without a matched sibling, ATG+CsA should be first-line therapy. In selected children with very severe disease, an unrelated donor (UD) graft may be considered first-line therapy (dashed arrow). In patients aged 0 to 60 years, nonresponders (no resp) to ATG have several choices, depending on the performance status of the patient, the availability of an HLA-matched UD, and patient age. The options are an UD BMT, a second course of IST (ATG+CsA), or an alternative donor transplant (Alt Donor Tx, indicating haploidentical transplants or cord blood [CB] transplants). At older than 60 years of age, medical treatment is preferable over BMT. d+120 = day +120; EPAG, eltrombopag; HLA = Sib, HLA-identical sibling; Sib BMT, identical sibling transplantation; UD BMT, unrelated donor BMT.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/11/10.1182_blood-2016-08-693481/4/m_blood693481f2.jpeg?Expires=1767697789&Signature=27Y8iGP7Dc4xkJWJpj9SWG9VYtJODMH4iclGtmCHfTdZF1N~DLONFbcngpI1-4l813a2rKXFn41ehWWIZGyyFR~6fW5K-UnP67INIYAV~YK9RxaRf4CrAECRxK0Y~bWj3uLGh-WKKXzR7X4H1tZ~9g1mVoAHIPqFnv00A29QVJym6yCTeLBMMV-sRXoVjk6M0ehlij6v86iU~RFb5t2VW7SOgpyNw119W6056JINF6k9kpOjM7uC9yBb53u6PfJkrWEpHcSQ638QjgP2npUk6Haz8l6YAGEqIMqvqteneI81mhOKxln2bE50bLZHYJICMC1oihduPF0EzfnlE0Jg8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)