In this issue of Blood, Liu et al provide evidence that miR-125b may contribute to leukemogenesis by activating an autocrine loop involving VEGFA.1
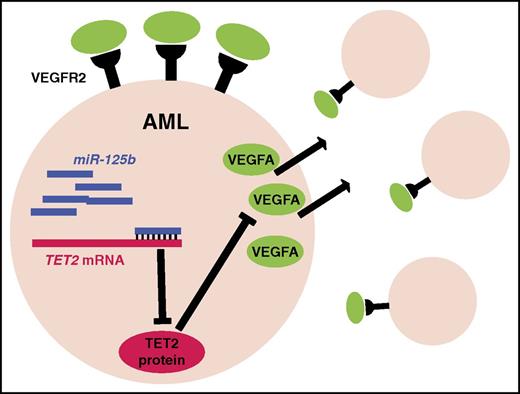
Model of the induction of a VEGFA autocrine loop by miR-125b. Overexpression of miR-125b inhibits translation of TET2, which in turn, through unclear mechanisms, results in increased VEGFA expression. VEGFA activates VEGFR2 on AML cells, resulting in increased survival and cellular proliferation.
Model of the induction of a VEGFA autocrine loop by miR-125b. Overexpression of miR-125b inhibits translation of TET2, which in turn, through unclear mechanisms, results in increased VEGFA expression. VEGFA activates VEGFR2 on AML cells, resulting in increased survival and cellular proliferation.
The miR-125 family contains 3 genes (MIR125A, MIR125B1, and MIR125B2) that are located on different chromosomes but share identical seed sequences. These genes are among the most highly expressed microRNAs in hematopoietic stem cells (HSCs), with expression levels declining during hematopoietic maturation. There is substantial evidence implicating miR-125 in the regulation of HSC function. Enforced expression of miR-125 family members enhances HSC engraftment and self-renewal activity and promotes skewing toward the myeloid lineage.2 Indeed, expression of miR-125a confers long-term repopulating activity on human and mouse multipotent progenitors.3
There is evidence implicating miR-125b in the pathogenesis of hematologic malignancies. miR-125b is highly expressed in a subset of acute myeloid leukemia (AML), including those with t(2:11)(p21;q23), AML1/ETO, and PML/RARα translocations. High miR-125b expression has also been observed in myelodysplastic syndrome with 5q deletions and acute megakaryoblastic leukemia associated with Down syndrome. Enforced expression of miR-125b in mice typically results in a myeloproliferative disorder characterized by leukocytosis, anemia, splenomegaly, and eventual death. Moreover, overexpression of miR-125b cooperates with BCR-ABL to induce a rapidly fatal myeloproliferative disease in mice.4 However, the mechanism(s) by which miR-125b cooperates with other oncogenes to promote leukemia initiation and maintenance remains poorly understood.
In the current study, Liu and colleagues developed a new transgenic mouse model with doxycycline-inducible miR-125b expression to explore the mechanisms through which miR-125b cooperates with other oncogenes to induce AML. They focused on the MLL-AF9 oncogene because miR-125b is highly expressed in a subset of AMLs with MLL translocations. Liu et al show that coexpression of miR-125b modestly shortens the latency of MLL-AF9–induced AML and increases the penetrance of AML after secondary transplantation. The latter effect was partially dependent on continued miR-125b overexpression, suggesting that miR-125b contributes to leukemia maintenance.
Initial studies to dissect the mechanism through which miR-125b cooperates with MLL-AF9 to induce AML yielded a surprise. In coculture experiments, Liu and colleagues noted that expression of miR-125b in AML cells stimulated, in trans, the proliferation of AML cells lacking the Mir125b transgene. This observation was confirmed in bone marrow chimeras containing both MLL-AF9 AML and MLL-AF9 AML with the Mir125b transgene. Induction of miR-125b in the latter induced expansion of both types of AML. These data suggest that miR-125b overexpression acts in part through a non–cell-intrinsic fashion to stimulate AML proliferation.
To identify potential mediators of this phenotype, the authors next surveyed cytokine expression in AML cells upon miR-125b overexpression. They noted a significant (12-fold) increase in VEGFA expression. Importantly, short hairpin RNA–mediated suppression of VEGFA expression in AML cells attenuated miR-125b–induced AML proliferation, as did treatment with a VEGFR2 inhibitor. An important caveat of this study is the lack of data showing that inhibition of VEGF signaling in vivo also suppresses miR-125b–induced AML proliferation. Nonetheless, the data suggest that miR-125b may contribute to leukemogenesis in part by inducing VEGFA expression. VEGFA is not a direct target of miR-125b. Rather, miR-125b appears to increase VEGFA expression in part by suppressing TET2, a known target of miR-125b. Of note, in human AML samples, VEGFA expression positively correlates with miR-125b expression, suggesting that the upregulation of VEGFA by miR-125b is relevant for human disease.
The major novel finding of this study, that miR-125b may contribute to leukemogenesis through the activation of a VEGFA-dependent autocrine loop (see figure), raises several questions and has important clinical implications. Both cell-intrinsic and non–cell-intrinsic mechanisms contribute to miR-125b’s effect on AML growth, and the importance of VEGFA signaling in mediating this effect remains unclear. Indeed, previous studies have provided evidence that miR-125b contributes to transformation by directly targeting TP53 or by targeting TNFAIP3, thereby activating the NF-κB pathway.5,6 In vivo pharmacologic or genetic approaches to attenuate VEGF signaling are needed to assess the importance of increased VEGFA signaling in miR-125b–induced leukemogenesis. Of note, recent clinical trials of VEGF inhibitors in AML have been disappointing. It will be interesting to determine whether the subset of AML that overexpresses miR-125b shows a better therapeutic response to VEGF inhibitors. Likewise, as miR-125b is proposed to promote VEGFA signaling at least partially through its repression of TET2, AMLs harboring mutations in TET2 may also be amenable to anti-VEGF therapy. Intriguingly, miR-125b has also been implicated in the pathogenesis of several nonhematopoietic malignancies, including breast cancer, ovarian cancer, and hepatocellular carcinoma, among others (reviewed in Sun et al7 ). Whether enhanced VEGFA signaling contributes to the neoplastic transformation or propagation of these tumors is an open question.
Conflict-of-interest disclosure: The authors declare no competing financial interests.