Key Points
MOF acetyltransferase activity is essential for adult but not early and midgestational murine hematopoietic maintenance.
Abstract
K(lysine) acetyltransferase 8 (KAT8, also known as MOF) mediates the acetylation of histone H4 at lysine 16 (H4K16ac) and is crucial for murine embryogenesis. Lysine acetyltransferases have been shown to regulate various stages of normal hematopoiesis. However, the function of MOF in hematopoietic stem cell (HSC) development has not yet been elucidated. We set out to study the role of MOF in general hematopoiesis by using a Vav1-cre–induced conditional murine Mof knockout system and found that MOF is critical for hematopoietic cell maintenance and HSC engraftment capacity in adult hematopoiesis. Rescue experiments with a MOF histone acetyltransferase domain mutant illustrated the requirement for MOF acetyltransferase activity in the clonogenic capacity of HSCs and progenitors. In stark contrast, fetal steady-state hematopoiesis at embryonic day (E) 14.5 was not affected by homozygous Mof deletion despite dramatic loss of global H4K16ac. Hematopoietic defects start manifesting in late gestation at E17.5. The discovery that MOF and its H4K16ac activity are required for adult but not early and midgestational hematopoiesis supports the notion that multiple chromatin regulators may be crucial for hematopoiesis at varying stages of development. MOF is therefore a developmental-stage–specific chromatin regulator found to be essential for adult but not early fetal hematopoiesis.
Introduction
Histone acetylation was first reported in 1964.1 More recently, histone acetyltransferases (HATs) have been shown to acetylate various nonhistone substrates; thus, HATs are now categorized as lysine acetyltransferases (KATs).2 KATs play key roles in normal and malignant hematopoiesis.3 Acetyltransferases such as p300, CBP, MOZ GCN5, and HBO1 were shown to regulate various stages of normal blood cell development, including hematopoietic stem cell (HSC) maintenance, myeloid proliferation, B-cell apoptosis, and erythropoiesis.4-8
KATs are divided into 5 families according to their homology and mechanism of acetylation, among which the MYST family is the largest.9 All members of the MYST family contain a MYST region with a canonical acetyl coenzyme A (CoA) binding site and a C2HC-type zinc finger motif.10 One of the best-characterized MYST-family proteins is K(lysine) acetyltransferase 8 (KAT8) also known as MOF. MOF mediates the acetylation of histone H4 at lysine 16 (H4K16ac) 11-13 and is crucial for murine embryogenesis.13,14 Murine embryos with homozygous constitutional loss of Mof do not develop past the blastocyst stage. MOF is a cell type–dependent regulator of chromatin state and controls various essential cellular processes such as DNA damage response,15-19 cell cycle progression,15,20 and embryonic stem cell self-renewal and pluripotency.21
MOF was shown to functionally and physically interact with the histone methyltransferase mixed-lineage leukemia 1 (MLL1).22 In hematopoiesis, MLL1 is essential for development and maintenance of both embryonic and adult progenitors and HSCs.23,24 However, its methyltransferase activity was recently shown to be dispensable for HSC maintenance and functionality.25 These findings prompt the question of whether MOF and its HAT activity are required for hematopoiesis. In 2013, Gupta et al26 showed that T-cell–specific deletion of Mof blocks differentiation and reduces T-cell numbers. To assess the role of MOF in hematopoietic development and HSC maintenance and differentiation, we used a conditional murine system14 in which the Vav1 promoter drives early embryonic hematopoietic expression of Cre recombinase27 (Vav1-cre;Moff/f mice). We found that MOF is critical for hematopoiesis in newborn and adult mice. In addition, we show that MOF acetyltransferase activity is required for HSC and progenitor maintenance and colony-forming capacity. However, MOF and H4K16ac seem dispensable for maintenance of the highly proliferative embryonic day 14.5 (E14.5) fetal hematopoietic system. Together, our findings illustrate that MOF-controlled chromatin regulation is a developmental-stage–specific mechanism from late gestational to adult hematopoietic maintenance.
Methods
Mice
The generation of a Mof conditional knockout mouse in a C57Bl/6 (CD45.2+) background has been described previously.14 Moff/f mice were crossed to Vav1-cre or Mx1-cre mice and Cre was maintained as a heterozygous allele. Genotyping strategies were described previously.14 Wild-type (WT) B6.SJL (CD45.1+) mice were purchased from Taconic Biosciences (Hudson, NY). All animal experiments in this study were approved by and adhered to guidelines of the Memorial Sloan Kettering Cancer Center Animal Care and Use Committee.
Isolation of murine hematopoietic cells
Vav1-cre;Moff/f, Vav1-cre;Moff/+, Moff/f, Mx1-cre;Moff/f, Mx1-cre, and WT adult mice or 8- to 9-day-old pups were euthanized, and peripheral blood (PB), spleen, thymus, liver, and bones (femurs, tibias, fibulas, pelvis, and spine) were extracted. Bone marrow (BM) cell suspensions were prepared by crushing bones. Spleen and thymus cell suspensions were prepared by crushing tissue through a 0.45-μm filter.
Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f fetal liver (FL) cells were obtained by timed breeding of a Vav1-cre;Moff/+and Moff/f couple and checking the females for a vaginal plug each day. Fourteen or 17 days after a vaginal plug was observed, pregnant females were euthanized (E14.5 or E17.5, respectively). Fetuses were extracted from the euthanized mother, and FL cell suspensions were prepared by flushing the liver through a 23-gauge needle in a tube containing phosphate-buffered saline with 2.5% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA).
Transplant experiments
All transplant experiments were performed by retro-orbitally injecting freshly isolated BM or FL cells into lethally irradiated (2 × 5 Gy), 6- to 8-week-old B6.SJL female recipient mice. Mice were euthanized at or after 16 weeks or euthanized sooner if they were severely anemic. Mice were bled once every 4 weeks to monitor complete blood count, percent of CD45.2 and mature hematopoietic populations. Further details on transplant experiments can be found in the supplemental Data, available on the Blood Web site.
In vitro colony-forming assays
To study the impact of Mof deletion on colony-forming capacity, fresh CD45.2+ sorted BM cells or whole FL cells (20 000 cells per dish for both) were plated in methylcellulose M3434 (STEMCELL Technologies, Vancouver, BC, Canada). Colonies were scored after 10 days by using an Eclipse TS100 inverted microscope (Nikon, Tokyo, Japan). Cells from pooled colony aggregates were then assessed for Mof excision.
For the Mof Lin–SCA1+cKIT+ (LSK) rescue experiments, fresh LSK cells from 4 Moff/f and 4 WT mice were pooled by genotype, split into 4 aliquots and each aliquot was infected with retrovirus containing miCD2, miCD2-Mof, miCD2-Mof-CoAdel, or miCD2-Mof-G327E. After 48 hours of liquid culture, cells were sorted for hCD2 positivity and infected with dTomato-Cre. Another 48 hours later, dTomato-positive cells were sorted, counted, and plated (7000 cells per dish) in M3434 methylcellulose.
Data analysis and statistical methods
GraphPad Prism software was used for statistical analysis. Statistical significance between 2 groups was determined by unpaired two-tailed Student t test. The Kaplan-Meier method was used to plot survival curves. More details are available in the supplemental Data.
Results
Homozygous Mof loss leads to lethal hematopoietic failure in mice at an early postnatal stage
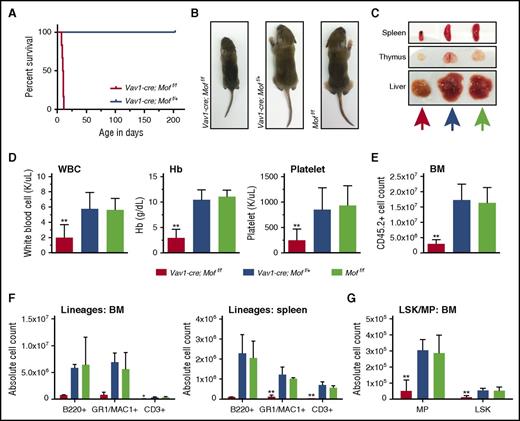
To examine the function of Mof in hematopoiesis, we used a conditional murine system14 in which the Vav1 promoter drives hematopoietic expression of Cre recombinase.27 Moff/f mice were backcrossed to C57Bl/6 mice.14 Mice with homozygous Mof loss in the hematopoietic compartment (Vav1-cre;Moff/f) were carried to term and were born with Mendelian frequencies, but they died 8 to 11 days after birth (P8-P11) whereas heterozygous Mof loss (Vav1-cre;Moff/+) did not affect survival (Figure 1A). At time of death, Vav1-cre;Moff/f P9 pups were smaller than both the heterozygous and WT (Moff/f) control (Figure 1B), had a smaller spleen and thymus (Figure 1C; supplemental Figure 1A), were pancytopenic (Figure 1D), and had low BM cellularity (Figure 1E). Flow cytometry (fluorescence-activated cell sorter [FACS]) analysis indicated that the absolute number of mature myeloid, lymphoid, and erythroid cells was significantly reduced in Vav1-cre;Moff/f P9 pups (Figure 1F; supplemental Figure 1B-C). A similar pattern was found in the T-cell lineage in thymoid tissue (supplemental Figure 1D-E). Total number of myeloid progenitors (MPs; Lin–SCA1–cKIT+) and LSK cells in Vav1-cre;Moff/f P9 BM were also significantly lower (Figure 1G). Within the BM compartment, the relative number of mature myeloid and lymphoid cells (supplemental Figure 1F) and erythroid cells (data not shown) remained similar, whereas the percentage of LSKs and MPs was significantly reduced in Vav1-cre;Moff/f P9 pups (supplemental Figure 2A-B). Together, these data show that homozygous Mof loss leads to lethal hematopoietic failure in mice shortly after birth and that although cell numbers in all lineages are affected, HSC-enriched populations may suffer the greatest losses.
Vav1-cre–induced homozygous Mof loss leads to lethal hematopoietic failure in mouse pups. (A) Survival curve of Vav1-cre;Moff/f and Vav1-cre;Moff/+ mice represented as days after birth (x-axis). (B) Representative photographs of 9-day-old Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f pups. (C) Representative photographs of spleen, thymus, and liver of 9-day-old Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f pups. Colors of arrows correlate to mouse genotype (Vav1-cre-Moff/f, orange; Vav1-cre-Moff/+, mustard green; Moff/f, blue). (D) White blood cell (WBC) count, hemoglobin (Hb), and platelet count at time of euthanasia (at 8-9 days of age). (E) BM cell count after CD45.2 selection. (F) Bar graphs representing the total number of mature B cells (B220+), myeloid cells (GR1+/MAC1+), and T cells (CD3+) in BM (harvested from femurs, pelvic bones, tibias, and spine) and spleen as measured by FACS. (G) Bar graph representing the total number of MP and LSK cells in BM as measured by FACS. Error bars represent standard deviation (SD) of the mean. Significance is shown for comparing Vav1-cre;Moff/f and Vav1-cre;Moff/+ to Moff/f. *P < .05; **P < .01.
Vav1-cre–induced homozygous Mof loss leads to lethal hematopoietic failure in mouse pups. (A) Survival curve of Vav1-cre;Moff/f and Vav1-cre;Moff/+ mice represented as days after birth (x-axis). (B) Representative photographs of 9-day-old Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f pups. (C) Representative photographs of spleen, thymus, and liver of 9-day-old Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f pups. Colors of arrows correlate to mouse genotype (Vav1-cre-Moff/f, orange; Vav1-cre-Moff/+, mustard green; Moff/f, blue). (D) White blood cell (WBC) count, hemoglobin (Hb), and platelet count at time of euthanasia (at 8-9 days of age). (E) BM cell count after CD45.2 selection. (F) Bar graphs representing the total number of mature B cells (B220+), myeloid cells (GR1+/MAC1+), and T cells (CD3+) in BM (harvested from femurs, pelvic bones, tibias, and spine) and spleen as measured by FACS. (G) Bar graph representing the total number of MP and LSK cells in BM as measured by FACS. Error bars represent standard deviation (SD) of the mean. Significance is shown for comparing Vav1-cre;Moff/f and Vav1-cre;Moff/+ to Moff/f. *P < .05; **P < .01.
Vav1-cre;Moff/f P9 hematopoietic BM cells are functionally impaired
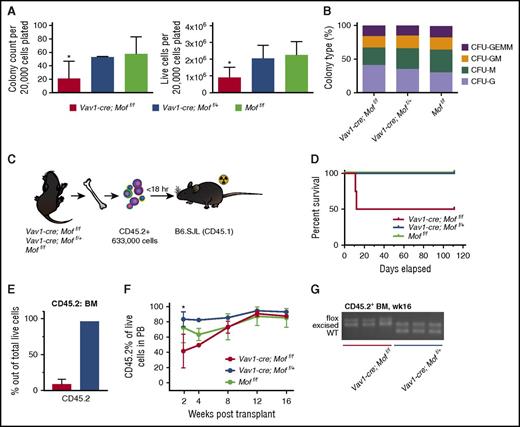
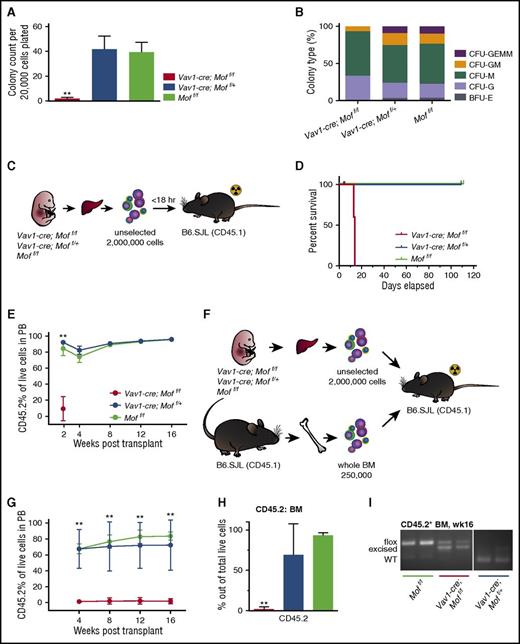
To test the functionality of P9 Vav1-cre;Moff/f BM MPs and HSCs, colony-forming capacity was tested in a colony-forming unit (CFU) assay. Figure 2A illustrates a significant but variable reduction in colony count and total number of live cells in Vav1-cre;Moff/f BM cells compared with Vav1-cre;Moff/+ or Moff/f BM cells. The types of colonies generated by Vav1-cre;Moff/f BM cells did not differ significantly (Figure 2B). Next, we performed noncompetitive transplant experiments in which 633 000 Vav1-cre;Moff/f, Vav1-cre;Moff/+, or Moff/f CD45.2+ BM cells were injected into lethally irradiated B6.SJL (CD45.1+) mice (Figure 2C). Two of 4 Vav1-cre;Moff/f recipients had to be sacrificed within 12 days posttransplant (Figure 2D) because of failure of donor cells to give rise to the minimally required hematopoiesis to survive lethal irradiation. Figure 2E illustrates the low percentage of donor cells in the recipient BM of the Vav1-cre;Moff/f recipient mice that failed to engraft compared with a properly engrafted Vav1-cre;Moff/+ recipient mouse (8.8% vs 96.5%). CD45.2 percentages of all surviving recipients were monitored (Figure 2F), and mice were euthanized 16 weeks posttransplant, at which time BM engraftment was ∼90% in all groups (data not shown). Polymerase chain reaction (PCR) analysis of Mof excision demonstrated incomplete excision in CD45.2+ BM cells derived from Vav1-cre;Moff/f recipients, whereas the floxed allele was completely excised in CD45.2+ BM cells from heterozygous recipients (Figure 2G), indicating that complete homozygous deletion of Mof is not compatible with engraftment. Overall, these functional experiments showed a variable phenotype of hematopoietic Vav1-cre;Moff/f cells. However, excision PCR data (Figure 2G) suggest that this variability is the result of high selection pressure against complete excision of Mof, which indicates that complete homozygous excision is not compatible with functional engraftment of postnatal hematopoietic cells in a noncompetitive transplant setting.
Vav1-cre-Moff/fP9 hematopoietic bone marrow cells are functionally impaired. (A) Day 10 of CFU assay of fresh, CD45.2+Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f P9 BM cells. Bar graphs indicate mean number of colonies after 10 days (left) and number of live cells (right) per plating. In all, 20 000 cells were plated per dish. Data are representative of 3 individual experiments with multiple donor mice per experiment. (B) Mean percentage of the various colony types relative to all colonies counted per dish per genotype. (C) Schematic for transplant experiment with CD45.2+Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f P9 BM cells. B6.SJL recipient mice were injected with BM cells shortly after lethal irradiation (2 × 5 Gy). The experiment was repeated 3 times with multiple donor mice per genotype per experiment. (D) Survival curve of recipient mice. Mice were all euthanized 16 weeks (wks) posttransplant. Vav1-cre;Moff/f, n = 4; Vav1-cre;Moff/+, n = 8; Moff/f, n = 9. (E) Bar graph illustrating the percentage of donor cells (CD45.2+) present in recipient BM comparing the Vav1-cre;Moff/f recipient mice (for which proper engraftment failed) to a properly engrafted Vav1-cre;Moff/+ recipient mouse. (F) Percent of CD45.2 live cells in PB over the time-course of the transplant experiments. (G) PCR analysis illustrating Mof excision in CD45.2+ BM cells at time of euthanasia. A representative image is shown. Error bars represent SD of mean. Significance is shown for comparing Vav1-cre;Moff/f and Vav1-cre;Moff/+ to Moff/f. *P < .05. CFU-GEMM, CFU–granulocyte, erythroid, macrophage, megakaryocyte; CFU-GM, CFU–granulocyte, macrophage; CFU-M, CFU-macrophage; CFU-G, CFU-granulocyte; BFU-E, burst forming unit-erythroid.
Vav1-cre-Moff/fP9 hematopoietic bone marrow cells are functionally impaired. (A) Day 10 of CFU assay of fresh, CD45.2+Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f P9 BM cells. Bar graphs indicate mean number of colonies after 10 days (left) and number of live cells (right) per plating. In all, 20 000 cells were plated per dish. Data are representative of 3 individual experiments with multiple donor mice per experiment. (B) Mean percentage of the various colony types relative to all colonies counted per dish per genotype. (C) Schematic for transplant experiment with CD45.2+Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f P9 BM cells. B6.SJL recipient mice were injected with BM cells shortly after lethal irradiation (2 × 5 Gy). The experiment was repeated 3 times with multiple donor mice per genotype per experiment. (D) Survival curve of recipient mice. Mice were all euthanized 16 weeks (wks) posttransplant. Vav1-cre;Moff/f, n = 4; Vav1-cre;Moff/+, n = 8; Moff/f, n = 9. (E) Bar graph illustrating the percentage of donor cells (CD45.2+) present in recipient BM comparing the Vav1-cre;Moff/f recipient mice (for which proper engraftment failed) to a properly engrafted Vav1-cre;Moff/+ recipient mouse. (F) Percent of CD45.2 live cells in PB over the time-course of the transplant experiments. (G) PCR analysis illustrating Mof excision in CD45.2+ BM cells at time of euthanasia. A representative image is shown. Error bars represent SD of mean. Significance is shown for comparing Vav1-cre;Moff/f and Vav1-cre;Moff/+ to Moff/f. *P < .05. CFU-GEMM, CFU–granulocyte, erythroid, macrophage, megakaryocyte; CFU-GM, CFU–granulocyte, macrophage; CFU-M, CFU-macrophage; CFU-G, CFU-granulocyte; BFU-E, burst forming unit-erythroid.
Mx1-cre–induced homozygous Mof loss in adult mice results in dramatic hematopoietic failure
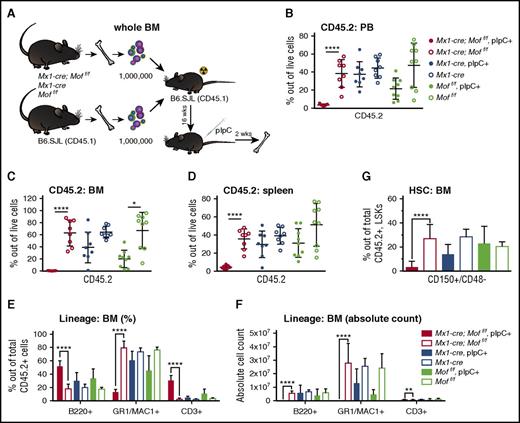
Because Vav1-cre;Moff/f mice did not live past the first 2 weeks of life, we used the Mx1-cre system to assess the effect of Mof loss on adult hematopoiesis. In Mx1-cre mice, the Mx1 promoter drives expression of Cre and can be induced by polyinosinic-polycytidylic acid (pIpC). In adult Mx1-cre;Moff/f and Mx1-cre;Moff/+ mice, induction of Cre by pIpC injections resulted in rapid and lethal pancytopenia in Mx1-cre;Moff/f mice (data not shown). Although Mx1 is expressed not only in hematopoietic tissue, we used a competitive transplant model to better assess the fate of adult hematopoietic cells upon MOF loss. Whole BM cells derived from an Mx1-cre;Moff/f, Mx1-cre, or Moff/f mouse were mixed at a 1:1 ratio with whole BM cells from a WT B6.SJL mouse and injected into lethally irradiated B6.SJL recipients (Figure 3A). After 16 weeks, the mean CD45.2 percentage in the PB was 26% in Mx1-cre;Moff/f recipients, 37% in Mx1-cre recipients, and 44% in Moff/f recipients (data not shown). Per genotype, mice were split in 2 even groups with a similar mean CD45.2 percentage, and 1 group was injected with pIpC. Two weeks after the last dose, mice were euthanized and processed. Figure 3B-D shows the CD45.2 percentage of live cells in PB, BM, and spleen, illustrating that homozygous Mof loss leads to a rapid and nearly complete loss of adult hematopoietic cells. Although mature myeloid cells are relatively more depleted in Mx1-cre;Moff/f pIpC-positive CD45.2+ BM cells than in mature lymphoid cells (Figure 3E), it becomes evident from the absolute cell counts (Figure 3F) that all lineages within the CD45.2+ compartment are dramatically depleted. A similar pattern was observed for the few residual CD45.2+ cells in PB and spleen (supplemental Figure 3A-B). FACS analysis for progenitors and HSCs showed significant depletion of MPs, LSKs, and long-term HSCs relative to the total number of CD45.2+ cells in Mof-deficient BM cells (Figure 3G; supplemental Figure 3C-D). Our findings indicate that Mof is required not only to maintain postnatal hematopoiesis but also to maintain adult hematopoiesis. Similar to what was found in postnatal hematopoiesis, cell numbers in all lineages are strongly affected by Mof loss in an adult transplant setting, but HSCs and progenitors suffer the greatest losses.
Mx1-cre induced homozygous Mof loss in adult mice results in dramatic hematopoietic failure. (A) Schematic for competitive transplant experiment with whole BM cells derived from adult Mx1-cre;Moff/f, Mx1-cre, and Moff/f mice and BM cells from a B6.SJL mouse. B6.SJL recipient mice were injected with fresh BM cells shortly after lethal irradiation. After 16 weeks, half the mice per genotype were injected with pIpC and 2 weeks after the fifth dose of pIpC, mice were euthanized and processed. The experiment was performed with 2 donors per genotype and 16 to 18 recipients per group. (B) Percent of CD45.2 live cells in PB of mice at time of euthanasia after pIpC treatment. Each dot represents a single mouse in the experiment. (C) Percent of CD45.2 live cells in BM of mice at time of euthansia after pIpC treatment. (D) Percent of CD45.2 live cells in spleen of mice at time of euthanasia after pIpC treatment. (E) Percentages of mature B cells (B220+), myeloid cells (GR1+/MAC1+), and T cells (CD3+) in live CD45.2+ BM cells as measured by FACS at time of euthanasia. (F) The total number of CD45.2+ mature B cells, myeloid cells, and T cells as measured by FACS at time of euthanasia. (G) Percentages of long-term HSCs (CD150+/CD48–) within the live CD45.2+ LSK BM population as measured by FACS at time of euthanasia. *P = .0006; **P = .0001; ****P < .000006. Error bars represent SD of mean.
Mx1-cre induced homozygous Mof loss in adult mice results in dramatic hematopoietic failure. (A) Schematic for competitive transplant experiment with whole BM cells derived from adult Mx1-cre;Moff/f, Mx1-cre, and Moff/f mice and BM cells from a B6.SJL mouse. B6.SJL recipient mice were injected with fresh BM cells shortly after lethal irradiation. After 16 weeks, half the mice per genotype were injected with pIpC and 2 weeks after the fifth dose of pIpC, mice were euthanized and processed. The experiment was performed with 2 donors per genotype and 16 to 18 recipients per group. (B) Percent of CD45.2 live cells in PB of mice at time of euthanasia after pIpC treatment. Each dot represents a single mouse in the experiment. (C) Percent of CD45.2 live cells in BM of mice at time of euthansia after pIpC treatment. (D) Percent of CD45.2 live cells in spleen of mice at time of euthanasia after pIpC treatment. (E) Percentages of mature B cells (B220+), myeloid cells (GR1+/MAC1+), and T cells (CD3+) in live CD45.2+ BM cells as measured by FACS at time of euthanasia. (F) The total number of CD45.2+ mature B cells, myeloid cells, and T cells as measured by FACS at time of euthanasia. (G) Percentages of long-term HSCs (CD150+/CD48–) within the live CD45.2+ LSK BM population as measured by FACS at time of euthanasia. *P = .0006; **P = .0001; ****P < .000006. Error bars represent SD of mean.
Homozygous Mof loss does not affect fetal hematopoiesis at E14.5
Essentially all definitive blood cells and a significant proportion of yolk sac–derived primitive blood cells express Vav1.27 In the embryo, Vav1 expression appears first in the liver, where it becomes just detectable at E9.5 and stronger at E12.5. At that stage, the liver has begun to replace the yolk sac and aorta-gonad-mesonephros system as the major hematopoietic organ.28 At E14.5, hematopoiesis is at its peak in the liver, after which it starts to divert to the BM. At E18, most of the hematopoietic progenitors are still located in the liver, but they gradually decrease through P14 and, already at P4, the majority of hematopoietic progenitors are found in the BM compartment.29
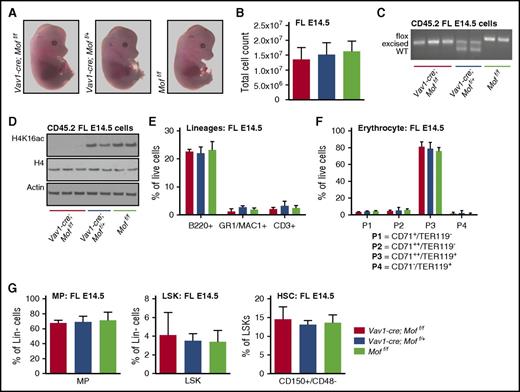
Because Vav1-cre;Moff/f pups were delivered to term but displayed lethal hematopoietic defects about a week after birth, we set out to study the effects of Mof loss on hematopoiesis in murine embryos at E14.5 when FL hematopoiesis is at its peak. The appearance of Vav1-cre;Moff/f embryos did not differ from their heterozygous and WT siblings (Figure 4A), and FL cellularity was consistent over all 3 genotypes (Figure 4B). PCR analysis on fresh CD45.2+ FL cells illustrated complete Mof excision of 1 or both alleles in Vav1-cre;Moff/f or Vav1-cre;Moff/+, respectively (Figure 4C), and quantitative reverse transcriptase PCR data show substantial downregulation of Mof in the homozygous knockout cells (supplemental Figure 4A). Consistent with homozygous Mof deletion, western blot analysis showed loss of global H4K16ac in CD45.2+Vav1-cre;Moff/f FL cells (Figure 4D). Despite this significant loss of H4K16ac, FACS analysis showed no change in lymphoid, myeloid, and erythroid lineages (Figure 4E-F), and MP, LSK, and HSC populations were also preserved (Figure 4G). These data indicate that Mof and subsequent H4K16ac loss does not affect steady-state fetal hematopoiesis at E14.5.
Vav1-cre–induced homozygous Mof loss does not affect fetal hematopoiesis at E14.5. (A) Representative photographs of Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos at E14.5. (B) Total live FL cell count. (C) PCR analysis illustrating Mof excision in fresh CD45.2+ FL cells. A representative image is shown. (D) Western blot showing global H4K16ac, H4, and actin in fresh CD45.2+ FL cells. (E) Percentages of mature B cells (B220+), myeloid cells (GR1+), and T cells (CD3+) in live FL cells as measured by FACS. (F) Percentages of cells at various stages of differentiation within the erythroid lineage in live FL cells as measured by FACS. (G) Percentages of MPs and LSKs in live, Lin– FL cells, and HSCs (CD150+/CD48–) as a percentage of LSKs, all measured by FACS. Error bars represent SD of mean.
Vav1-cre–induced homozygous Mof loss does not affect fetal hematopoiesis at E14.5. (A) Representative photographs of Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos at E14.5. (B) Total live FL cell count. (C) PCR analysis illustrating Mof excision in fresh CD45.2+ FL cells. A representative image is shown. (D) Western blot showing global H4K16ac, H4, and actin in fresh CD45.2+ FL cells. (E) Percentages of mature B cells (B220+), myeloid cells (GR1+), and T cells (CD3+) in live FL cells as measured by FACS. (F) Percentages of cells at various stages of differentiation within the erythroid lineage in live FL cells as measured by FACS. (G) Percentages of MPs and LSKs in live, Lin– FL cells, and HSCs (CD150+/CD48–) as a percentage of LSKs, all measured by FACS. Error bars represent SD of mean.
Vav1-cre;Moff/f hematopoietic E14.5 fetal liver cells are functionally impaired
CFU assays were performed to assess the functional capacity of E14.5 Vav1-cre;Moff/f FL HSCs and myeloid progenitors. The colony-forming capacity of Vav1-cre;Moff/f FL cells was greatly diminished (Figure 5A). The few colonies that formed were more mature (CFU-M and CFU-G; Figure 5B), suggesting that HSCs or early progenitors may be affected to a greater extent than more mature populations. Next, we performed 2 noncompetitive transplant experiments in which 2 000 000 Vav1-cre;Moff/f, Vav1-cre;Moff/+, or Moff/f unselected FL cells were injected into lethally irradiated B6.SJL mice (Figure 5C). All 6 Vav1-cre;Moff/f recipients had to be euthanized within 12 days posttransplant (Figure 5D) as a result of failure of donor cells to give rise to the minimally required hematopoiesis needed to survive lethal irradiation. Figure 5E shows the percentage of donor cells (CD45.2+) in the recipient PB. This indicates that Mof-deficient E14.5 FL cells were unable to reconstitute hematopoiesis in adult mice, whereas heterozygous Mof loss did not affect reconstitution. These findings are suggestive of a possible homing defect of FL cells to the BM and indeed, we found that Vav1-cre;Moff/f cells exhibit impaired homing capacity in a transplant setting (supplemental Figure 4B-C).
Vav1-cre;Moff/fhematopoietic E14.5 fetal liver cells are functionally impaired. (A) Day 10 of CFU assay of fresh FL cells derived from E14.5 Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos. Bar graph indicates mean number of colonies per dish after 10 days. In all, 20 000 cells were plated per dish. Data are representative of 3 individual experiments with multiple embryos per experiment. (B) Shown is the mean percentage of the various colony types relative to all colonies counted per dish per genotype. (C) Schematic for transplant experiment with FL cells derived from E14.5 Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos. B6.SJL recipient mice were injected with FL cells shortly after lethal irradiation (2 × 5 Gy). The experiment was repeated 2 times with multiple donor embryos per genotype per experiment. (D) Survival curve of recipient mice. All mice were euthanized 16 weeks posttransplant. Vav1-cre;Moff/f, n = 6; Vav1-cre;Moff/+, n = 6; Moff/f, n = 7. (E) Percent of CD45.2 live cells in PB over the time-course of the transplant experiments. (F) Schematic for transplant experiment with FL cells derived from E14.5 Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos and 250 000 whole BM helper cells. B6.SJL recipient mice were injected with cells shortly after lethal irradiation. The experiment was repeated 2 times with multiple donor embryos per genotype per experiment. (G) Percent of CD45.2 live cells in the PB over the time-course of the transplant experiments. Vav1-cre;Moff/f, n = 6; Vav1-cre;Moff/+, n = 5; Moff/f, n = 5. (H) Percent of CD45.2 live cells in BM of recipient mice at week 16. (I) PCR analysis illustrating Mof excision in CD45.2+ BM cells at time of euthanasia. A representative image is shown. Error bars represent SD of mean. Significance is shown for comparing Vav1-cre;Moff/f and Vav1-cre;Moff/+ to Moff/f. **P < .01.
Vav1-cre;Moff/fhematopoietic E14.5 fetal liver cells are functionally impaired. (A) Day 10 of CFU assay of fresh FL cells derived from E14.5 Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos. Bar graph indicates mean number of colonies per dish after 10 days. In all, 20 000 cells were plated per dish. Data are representative of 3 individual experiments with multiple embryos per experiment. (B) Shown is the mean percentage of the various colony types relative to all colonies counted per dish per genotype. (C) Schematic for transplant experiment with FL cells derived from E14.5 Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos. B6.SJL recipient mice were injected with FL cells shortly after lethal irradiation (2 × 5 Gy). The experiment was repeated 2 times with multiple donor embryos per genotype per experiment. (D) Survival curve of recipient mice. All mice were euthanized 16 weeks posttransplant. Vav1-cre;Moff/f, n = 6; Vav1-cre;Moff/+, n = 6; Moff/f, n = 7. (E) Percent of CD45.2 live cells in PB over the time-course of the transplant experiments. (F) Schematic for transplant experiment with FL cells derived from E14.5 Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos and 250 000 whole BM helper cells. B6.SJL recipient mice were injected with cells shortly after lethal irradiation. The experiment was repeated 2 times with multiple donor embryos per genotype per experiment. (G) Percent of CD45.2 live cells in the PB over the time-course of the transplant experiments. Vav1-cre;Moff/f, n = 6; Vav1-cre;Moff/+, n = 5; Moff/f, n = 5. (H) Percent of CD45.2 live cells in BM of recipient mice at week 16. (I) PCR analysis illustrating Mof excision in CD45.2+ BM cells at time of euthanasia. A representative image is shown. Error bars represent SD of mean. Significance is shown for comparing Vav1-cre;Moff/f and Vav1-cre;Moff/+ to Moff/f. **P < .01.
To determine short- and long-term engraftment potential of Mof-deficient E14.5 FL cells, the rapid radiation-induced death of recipient mice had to be circumvented. Therefore, we repeated the FL transplants, but now we co-injected 250 000 whole BM CD45.1+ WT helper cells (Figure 5F). Over the entire time course of the experiment, CD45.2 percentages did not rise above 2% in the PB of Vav1-cre;Moff/f FL recipients, whereas Vav1-cre;Moff/+ and Moff/f recipients demonstrated stable engraftment with CD45.2 of ∼75% (Figure 5G). At time of euthanasia (week 16 posttransplant), the mean CD45.2 percentage in BM of Mof-deficient FL recipients was 1.8%, which is significantly lower than that in Vav1-cre;Moff/+ (69.2%) or Moff/f (93.2%) recipients (Figure 5H). The few FL cells that did engraft in the Vav1-cre;Moff/f group were only partially excised, unlike the engrafted Vav1-cre;Moff/+ FL cells in which the 1 allele was completely excised (Figure 5I). FACS analysis of the CD45.2+ BM cells revealed no significant differences among the 3 groups with regard to the percentage of mature lymphocytes, myeloid cells (supplemental Figure 4D), LSKs, and long-term HSCs (supplemental Figure 4E).
In contrast to the presumably normal hematopoiesis in Vav1-cre;Moff/f embryos, these experiments demonstrated significant functional impairment of Mof-deficient E14.5 FL cells. However, these experiments address fetal HSC functionality outside the fetal microenvironment and therefore suggest an important role of the fetal microenvironment in maintaining normal hematopoiesis in Vav1-cre;Moff/f embryos.
Mof-deficient embryos start manifesting hematopoietic defects at E17.5
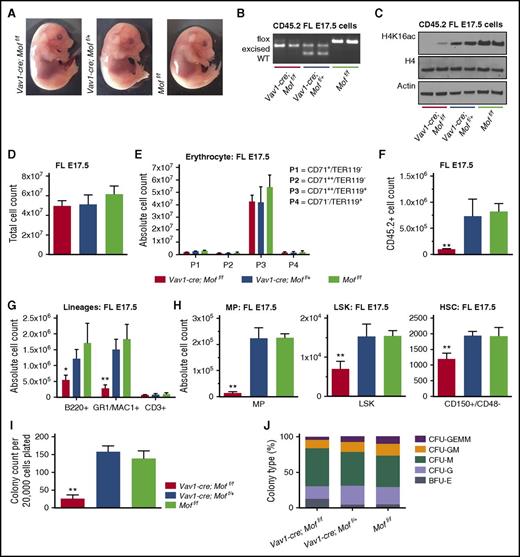
We wanted to further narrow down the time frame in which murine hematopoiesis starts to derail because of Mof deficiency. Given the lack of phenotype in Mof-deficient E14.5 hematopoiesis, we chose to assess FL hematopoiesis at E17.5, a late gestational age, only 1.5 days before birth. Gross morphology of Vav1-cre;Moff/f embryos seemed normal (Figure 6A), and the Mof allele was largely excised in hematopoietic Vav1-cre;Moff/f FL cells (Figure 6B), consistent with a loss of global H4K16ac (Figure 6C). Total FL cell counts were comparable among all 3 groups (Figure 6D), and erythroid populations did not seem to be affected by loss of Mof (Figure 6E), fitting with the normal appearance of Mof-null embryos. However, the CD45.2+ cell number was significantly decreased in E17.5 Vav1-cre;Moff/f livers (Figure 6F). FACS analysis of these Mof-deficient CD45.2+ FL cells indicated that the absolute number of mature myeloid and lymphoid cells (Figure 6G) and the number of MPs, LSKs, and HSCs (Figure 6H) was significantly reduced in Vav1-cre;Moff/f embryos. These Mof-deficient FL cells were also functionally impaired, and they formed fewer colonies than the heterozygous and WT control (Figure 6I). The types of colonies did not differ significantly (Figure 6J). Together these findings suggest that hematopoietic defects resulting from Mof and subsequent H4K16ac loss start manifesting in late gestation, although at E17.5, the phenotype is still quite modest compared with the phenotype in pups and adult mice. Interestingly, at E17.5, the erythroid lineage seems intact, which could explain why Mof-deficient embryos are born at normal ratios.
Mof-deficient embryos start manifesting hematopoietic defects at E17.5. (A) Representative photographs of Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos at E17.5. (B) PCR analysis illustrating Mof excision in fresh CD45.2+ FL cells. A representative image is shown. (C) Western blot showing global H4K16ac, H4, and actin in fresh CD45.2+ FL cells. (D) Total live FL cell count. (E) The absolute number of cells at various stages of differentiation within the erythroid lineage in E17.5 FLs as measured by FACS. (F) Total number of live CD45.2+ FL cells. (G) The absolute number of mature B cells (B220+), myeloid cells (GR1+), and T cells (CD3+) in E17.5 FL cells as measured by FACS. (H) The absolute number of MPs, LSKs, and HSCs (CD150+/CD48–) as measured by FACS. (I) Day 10 of CFU assay of fresh FL cells derived from E17.5 Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos. Bar graph indicates mean number of colonies per dish after 10 days. In all, 20 000 cells were plated per dish. Each groups contains 3 biological replicates. (J) Shown is the mean percentage of the various colony types relative to all colonies counted per dish per genotype. Error bars represent SD of mean. Significance is shown for comparing Vav1-cre;Moff/f and Vav1-cre;Moff/+ to Moff/f. *P < .05; **P < .01.
Mof-deficient embryos start manifesting hematopoietic defects at E17.5. (A) Representative photographs of Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos at E17.5. (B) PCR analysis illustrating Mof excision in fresh CD45.2+ FL cells. A representative image is shown. (C) Western blot showing global H4K16ac, H4, and actin in fresh CD45.2+ FL cells. (D) Total live FL cell count. (E) The absolute number of cells at various stages of differentiation within the erythroid lineage in E17.5 FLs as measured by FACS. (F) Total number of live CD45.2+ FL cells. (G) The absolute number of mature B cells (B220+), myeloid cells (GR1+), and T cells (CD3+) in E17.5 FL cells as measured by FACS. (H) The absolute number of MPs, LSKs, and HSCs (CD150+/CD48–) as measured by FACS. (I) Day 10 of CFU assay of fresh FL cells derived from E17.5 Vav1-cre;Moff/f, Vav1-cre;Moff/+, and Moff/f embryos. Bar graph indicates mean number of colonies per dish after 10 days. In all, 20 000 cells were plated per dish. Each groups contains 3 biological replicates. (J) Shown is the mean percentage of the various colony types relative to all colonies counted per dish per genotype. Error bars represent SD of mean. Significance is shown for comparing Vav1-cre;Moff/f and Vav1-cre;Moff/+ to Moff/f. *P < .05; **P < .01.
MOF histone acetyltransferase activity is required for adult hematopoietic cell survival
MOF has been identified as the major H4K16ac in humans, mice, and Drosophila.11-14,20 MOF contains a MYST family HAT domain with a CoA-binding site (Figure 7A) that was shown to be crucial for its acetyltransferase activity.10,12 Although MOF possesses acetyltransferase activity on various histones and nucleosomes, depletion of MOF in HeLa cells was shown to lead to a dramatic decrease in H4K16ac, whereas other acetylation sites seemed to be unaffected.20
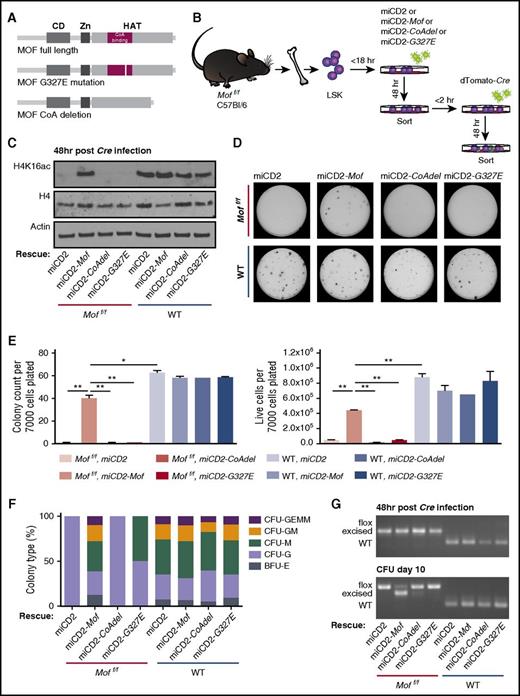
MOF histone acetyltransferase activity is required for adult hematopoietic cell survival. (A) Schematic of full-length MOF, CoA-binding domain deleted MOF, or G327E mutant MOF. (B) Schematic for in vitro rescue experiment. BM LSKs derived from 4 Moff/f or 4 WT mice were pooled by genotype and retrovirally transduced with full-length Mof (miCD2-Mof), a Mof mutant (miCD2-CoAdel or miCD2-G327E), or empty vector (miCD2), and hCD2+ cells were sorted after 48 hours. These cells were then infected with dTomato-Cre, sorted another 48 hours (hr) later and used for CFU assays. (C) Western blot showing global H4K16ac, H4, and actin in Moff/f and WT miCD2, miCD2-Mof, miCD2-Mof-CoAdel and miCD2-Mof-G327E cells at 48 hours after Cre transduction. (D) Day 10 of CFU assay. In all, 7000 cells were plated per dish. Representative petri dishes are shown. (E) Day 10 of CFU assay. Bar graph indicates mean number of colonies per dish after 10 days (left) or mean number of live cells (right) per dish. Data are representative of 2 individual experiments. (F) Mean percentage of the various colony types relative to all colonies counted per dish per genotype. (G) PCR analysis illustrating Mof excision in hCD2+, dTomato+ LSKs 48 hours after Cre infection and at day 10 of the colony-forming assay. A representative image is shown.
MOF histone acetyltransferase activity is required for adult hematopoietic cell survival. (A) Schematic of full-length MOF, CoA-binding domain deleted MOF, or G327E mutant MOF. (B) Schematic for in vitro rescue experiment. BM LSKs derived from 4 Moff/f or 4 WT mice were pooled by genotype and retrovirally transduced with full-length Mof (miCD2-Mof), a Mof mutant (miCD2-CoAdel or miCD2-G327E), or empty vector (miCD2), and hCD2+ cells were sorted after 48 hours. These cells were then infected with dTomato-Cre, sorted another 48 hours (hr) later and used for CFU assays. (C) Western blot showing global H4K16ac, H4, and actin in Moff/f and WT miCD2, miCD2-Mof, miCD2-Mof-CoAdel and miCD2-Mof-G327E cells at 48 hours after Cre transduction. (D) Day 10 of CFU assay. In all, 7000 cells were plated per dish. Representative petri dishes are shown. (E) Day 10 of CFU assay. Bar graph indicates mean number of colonies per dish after 10 days (left) or mean number of live cells (right) per dish. Data are representative of 2 individual experiments. (F) Mean percentage of the various colony types relative to all colonies counted per dish per genotype. (G) PCR analysis illustrating Mof excision in hCD2+, dTomato+ LSKs 48 hours after Cre infection and at day 10 of the colony-forming assay. A representative image is shown.
Because our data indicate a pivotal role for Mof in adult hematopoiesis, we set out to establish the specificity of our model for Mof loss and determine whether MOF HAT activity is dispensable. We designed a full-length, human influenza hemagglutinin (HA) -tagged Mof and two HAT inactivated Mof retroviral constructs (Figure 7A) in which either the CoA-binding site was deleted (Mof-CoAdel) or a HAT inactivating point mutation (G327E) was introduced (Mof-G327E). The HAT inactivating point mutation in the MOF CoA-binding domain was first described in Drosophila12 and later used in a human MOF construct where it was also found to diminish H4K16ac.30 Alignment of Drosophila, murine, and human MOF illustrates that the point mutation involves replacement of glycine by glutamic acid on position 327 in murine as well as human MOF (supplemental Figure 5A). The constructs were packaged in an miCD2-MSCV retroviral vector with the hCD2 selection marker (miCD2-Mof, miCD2-Mof-CoAdel, and miCD2-Mof-G327E). Our data on Mof loss in the postnatal stage indicate a variable phenotype, most likely the result of leakage of the Cre system and high selection pressure against excised cells (Figure 2G). We therefore set up an in vitro Cre transduction experiment using adult Moff/f or WT BM-derived LSKs. To assess expression of the exogenous Mof constructs, we performed western blot analysis for HA-tagged MOF in transformed mouse BM LSKs. Supplemental Figure 5B illustrates that exogenous Mof was consistently expressed by miCD2-Mof, miCD2-Mof-G327E, and miCD2-Mof-CoAdel.
For the adult BM-derived LSK rescue experiment, we transduced Moff/f or WT LSKs with miCD2, miCD2-Mof, miCD2-Mof-G327E, or miCD2-Mof-CoAdel. After 48 hours, cells were sorted and infected with dTomato-Cre. After another 48 hours, dTomato-positive cells were sorted, harvested for genomic DNA or protein, and plated in methylcellulose (Figure 7B). Western blot analysis confirmed that exogenous Mof was capable of restoring H4K16ac levels upon genetic Mof loss, whereas both HAT domain mutant Mof constructs were not (Figure 7C). CFU assays showed that exogenous full-length MOF could indeed rescue hematopoietic Cre-positive Moff/f cells from their detrimental fate, indicating the specificity of Mof loss in the observed phenotype (Figure 7D-E). However, HAT-deficient MOF was incapable of rescuing Cre-positive Moff/f cells (Figure 7D-E), and the few colonies that did form were differentiated (Figure 7F). These findings are supported by Mof excision PCR data in which consistent and strong excision was observed in Cre-positive Moff/fmiCD2-Mof cells throughout the experiment, whereas the few colonies that did grow in the Cre-positive Moff/fmiCD2, miCD2-Mof-CoAdel, and miCD2-Mof-G327E setting were largely unexcised by day 10 (Figure 7G).
Interestingly, we found that Mof-deficient LSKs had significantly more yH2AX foci per cell nucleus compared with the WT control (P < .01; supplemental Figure 5C-D), indicative of more DNA damage. All together these data indicate that the observed phenotype of homozygous Mof deletion in hematopoietic adult cells is specific to the loss of Mof. In addition, we have demonstrated that the HAT activity of MOF is crucial for survival of adult hematopoietic cells and that it may be required for genomic stability or adequate DNA damage response in adult HSCs.
Discussion
The discovery that MOF is required for adult but not early fetal hematopoiesis sheds new light on our understanding of regulation of hematopoietic development. We demonstrated that MOF is critical for hematopoietic cell maintenance and reconstitution in adult hematopoiesis. Rescue experiments with MOF HAT domain mutants illustrated the requirement for MOF acetyltransferase activity in the clonogenic capacity of HSCs and progenitors. In stark contrast, fetal steady-state hematopoiesis at E14.5 did not seem to be affected by homozygous Mof deletion despite dramatic loss of global H4K16ac. Given that previously published work using the Vav1-cre system has generated defects in fetal HSCs at E14.5,31 we conclude that MOF plays a critical role in adult but not early fetal HSC maintenance.
HSCs undergo discrete developmental changes throughout life, the biggest being the transition from fetal to adult hematopoiesis.32 Early and midgestational hematopoiesis is characterized by rapid proliferation of undifferentiated HSCs to supply the developing system.33 In adult hematopoiesis, lifelong blood cell production depends on the balance between HSC self-renewal and differentiation,34 so in contrast to fetal hematopoiesis, most adult HSCs turn quiescent35 to ensure HSC pool maintenance. This switch sets in at E16.5 and is complete by about 3 weeks after birth.32 Because fetal HSCs markedly differ from adult HSCs with respect to cell cycle status,35 proliferative capacity,33 and differentiation potential,36 it is not surprising that they also differ in gene expression31,37 and regulation.31,38 Although some genes such as Meis1,39,40 Rae28,41,42 and Myb43,44 regulate HSC maintenance throughout fetal and adult life, the transcription factor Sox1731 was found to be essential for fetal but not adult hematopoiesis. Similar to the observed phenotype in hematopoietic Mof loss, the transcriptional regulator Bmi1 has been shown to maintain adult but not early fetal HSCs.38 Although pluripotency and lineage differentiation depend on establishing and maintaining gene expression programs that are largely regulated by chromatin organization,45 it seems likely that multiple chromatin regulators are crucial for hematopoiesis at varying stages of development. Consistent with this notion is the finding that the chromatin regulator Ezh2 is required for fetal but not adult hematopoiesis.46 Our findings support this concept of chromatin regulators being differentially involved in hematopoietic development, whereas we have shown that MOF HAT activity is a crucial regulator of postnatal and adult hematopoiesis but not of the highly proliferative early and midgestational hematopoietic compartment. The fact that we can isolate fetal phenotypically defined HSCs that are completely devoid of H4K16ac is quite remarkable and suggests a dramatic difference in chromatin structure between fetal and adult HSCs.
Further highlighting the importance of H4K16 acetylation, we have shown that the enzymatic activity of MOF is crucial for adult HSC maintenance and functionality (Figure 7E). In addition we found an increase in DNA damage in Mof-deficient adult HSCs (supplemental Figure 5C-D). Future studies will be required to test why fetal hematopoietic cells can exist despite the dramatic MOF loss–induced H4K16ac deficiency (Figure 4D). A genome-wide distribution study of H4K16ac in HEK293 cells illustrated that H4K16ac has a limited effect on transcriptional regulation,47 whereas H4K16ac was found to be critical in regulating transcription in mouse embryonic stem cells.21 Although most studies of Mof depletion report adverse outcomes, a recent study48 found that Mof deficiency and concurrent H4K16ac loss in quiescent glomerular podocytes had no effect on kidney function. These findings suggest that H4K16ac requirement may indeed be cell type–specific, although it is surprising that a highly proliferative hematopoietic system such as that found in an E14.5 embryo can be maintained despite lack of H4K16ac. This is particularly interesting because H4K16ac is believed to be one of the most important histone modifications to maintain euchromatin in mammalian cells.49 Previous data from murine embryonic fibroblasts suggest that a MOF-induced increase of H4K16ac is essential for an appropriate DNA damage response.17 Our finding of DNA damage increase in Mof-deficient LSKs leads us to speculate that H4K16ac depletion in adult HSCs potentially reduces accessibility of nucleosomes to repair proteins by increasing chromatin compaction at the DNA damage foci, leading to gross genomic instability. Possibly other chromatin marks compensate for this lack of H4K16ac in fetal HSCs. Another possibility is that the regulation of chromatin structure is dramatically different between fetal and adult HSCs. Future studies using quantitative mass spectrometry to define global epigenomic changes50 could yield significant mechanistic insight into the role of altered epigenomic states throughout hematopoietic development.
The observed hematopoietic defects found in the liver of E17.5 Mof-deficient embryos (Figure 6), although more moderate than in pups and adults, make it unlikely that the phenotype in postnatal and adult Mof-null mice is solely caused by an impaired seeding capacity or engraftment of FL cells to the BM niche. Previous work has shown that distinct microenvironmental regulatory mechanisms exist between the fetal and adult HSC niche.32,33,51,52 However, it is conceivable that the FL microenvironment itself is also modified during fetal development to meet the changing needs of lineage differentiation and HSC expansion.32 Our data suggest that earlier embryonic Mof-deficient HSCs (<E15.5) are capable of proliferation and differentiation (Figure 4). However, over the next few days of gestation, intrinsic cellular requirements and microenvironmental stimuli change, and it may be that Mof-null, H4K16ac-deficient HSCs are incapable of adapting to these changes because of significant abnormalities in chromatin structure.
As previously mentioned, MOF and MLL1 complexes interact, and this interaction was shown to be important for the chromatin regulatory function of both enzymes.22 Apart from its role in normal hematopoiesis, WT MLL1 has been shown to play a role in MLL-rearranged leukemogenesis.53,54 However, in both normal and malignant hematopoiesis, the H3K4 methyltransferase activity of MLL1 is dispensable.25 Given the interaction of MLL1 and MOF, and the necessity of MOF HAT activity in adult hematopoiesis, it will be interesting to determine leukemia dependence on the enzymatic activity of MOF. It may be that in the setting of MLL-rearranged leukemia, it is in fact the HAT activity of MOF that is required for leukemogenesis. Future experiments assessing the role of MOF HAT activity in MLL-fusion leukemogenesis should shed light on this hypothesis.
In conclusion, our study shows that MOF is a critical regulator of adult but not early fetal hematopoiesis, thereby supporting the notion that chromatin regulation of hematopoiesis changes over time. We have identified MOF-controlled chromatin regulation as a developmental-stage–specific mechanism for adult hematopoietic maintenance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Z. Feng for administrative assistance.
This work was supported by a CURE Childhood Cancer Research Grant (D.G.V.), National Institutes of Health (NIH), National Cancer Institute grants P01 CA66996 and R01 CA140575 (S.A.A.), the Leukemia and Lymphoma Society (S.A.A.), Gabrielle’s Angel Research Foundation (S.A.A.), a Department of Defense Bone Marrow Failure Postdoctoral Fellowship Award (W81XWH-12-1-0568) (H.X.); NIH, National Cancer Institute grants R01 CA129537 and R01 CA154320 (T.K.P.); and an NIH Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748).
Authorship
Contribution: D.G.V., H.X., and S.A.A. conceived the study and designed the experiments; D.G.V., H.X., M.E.E., and C.M.W. performed experiments; D.G.V. performed all data analysis; T.K.P. generated the conditional Mof knockout mouse model; and D.G.V. and S.A.A. wrote the manuscript.
Conflict-of-interest disclosure: S.A.A. is a consultant for Epizyme Inc. and Imago Biosciences. The remaining authors declare no competing financial interests.
Correspondence: Scott A. Armstrong, Department of Pediatric Oncology, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215; e-mail: scott_armstrong@dfci.harvard.edu.
References
Author notes
D.G.V. and H.X. contributed equally to this work