Abstract
Aside from the established role for platelets in regulating hemostasis and thrombosis, recent research has revealed a discrete role for platelets in the separation of the blood and lymphatic vascular systems. Platelets are activated by interaction with lymphatic endothelial cells at the lymphovenous junction, the site in the body where the lymphatic system drains into the blood vascular system, resulting in a platelet plug that, with the lymphovenous valve, prevents blood from entering the lymphatic circulation. This process, known as “lymphovenous hemostasis,” is mediated by activation of platelet CLEC-2 receptors by the transmembrane ligand podoplanin expressed by lymphatic endothelial cells. Lymphovenous hemostasis is required for normal lymph flow, and mice deficient in lymphovenous hemostasis exhibit lymphedema and sometimes chylothorax phenotypes indicative of lymphatic insufficiency. Unexpectedly, the loss of lymph flow in these mice causes defects in maturation of collecting lymphatic vessels and lymphatic valve formation, uncovering an important role for fluid flow in driving endothelial cell signaling during development of collecting lymphatics. This article summarizes the current understanding of lymphovenous hemostasis and its effect on lymphatic vessel maturation and synthesizes the outstanding questions in the field, with relationship to human disease.
The lymphatic vascular system has many essential physiological functions, including maintenance of tissue fluid homeostasis by returning interstitial fluid to the blood, absorption of dietary lipids through mesenteric lymphatics, and trafficking of immune cells between lymph nodes. Dysfunction of the lymphatic system can lead to severe edema either congenitally or acquired later in life, and systemic absence of the lymphatic system due to genetic mutation is incompatible with life.1,2 Interestingly, platelets have an unexpected, yet critical, role in the separation of the blood and lymphatic systems and regulation of lymphatic flow.
An unexpected role for platelets in blood/lymphatic vessel separation
Lymphatic endothelial cell (LEC) specification occurs in a subset of venous endothelial cells in the cardinal vein at embryonic day E9.75 in the mouse when the critical transcription factor PROX1 is expressed.3,4 These PROX1-expressing LEC progenitors then bud off the cardinal vein, following a vascular endothelial growth factor receptor-C gradient, to form lymph sacs that eventually constitute most of the lymphatic vascular system throughout the body2,5 (for a thorough review of lymphatic development, see Aspelund et al6 ). As LECs migrate out of the cardinal vein to form nascent lymph sacs, the 2 vascular systems remain connected, and the exclusion of blood cells from the lymphatic system is important. Surprisingly, platelets play a critical role in prevention of blood cells entering the developing lymphatic vessels, a process known as lymphovenous hemostasis.
The first evidence of a role for hematopoietic cells in separation of the blood and lymphatic vascular systems came in mice that were deficient in Slp-76 or Syk, proteins that mediate signaling in several hematopoietic lineages.7 These mice exhibited embryonic blood-filled lymphatic vessels and perinatal lethality due to chylothorax/chylous ascites.7-9 Importantly, blood vessel hemorrhage was not responsible for the appearance of blood in the lymphatic vessels, and it was proposed that there might exist aberrant connections between the blood and lymphatic vessels.7 At this time, the mechanism whereby deletion of intracellular hematopoietic cell signaling proteins directed proper separation of the blood and lymphatic vascular systems was unclear. A critical clue was found by Suzuki-Inoue et al, who reported that podoplanin (T1α), an LEC marker that is not expressed on blood endothelial cells, is the ligand that binds to C-type Lectin Receptor 2 (CLEC-2), which is expressed on platelets and contains a signaling motif capable of activating SYK and Slp-76 signaling.10 Interaction of podoplanin with CLEC-2 provided a connection by which a hematopoietic cell signaling pathway could interact with LECs.10 Subsequently, a critical role for podoplanin/CLEC-2 interaction in blood/lymphatic separation was identified in mice lacking O-glycosylation required for podoplanin synthesis as well as podoplanin and CLEC-2 knockout mice, all of which exhibited lymphatic vessel defects and blood/lymphatic mixing similar to the Syk/Slp-76 phenotypes.11-13 Conditional knockout mice using the platelet-specific PF4-Cre and bone marrow transplant experiments confirmed that CLEC-2 and Slp76 are required specifically in platelets, and podoplanin is required in nonhematopoietic cells, to mediate platelet aggregation on LECs and blood/lymphatic separation.9,14,15
Collectively, these studies have defined a critical signaling pathway that mediates separation of the blood and lymphatic vascular systems during development. As LECs specify from venous endothelial cells, and migrate out of the cardinal vein, podoplanin is expressed and O-glycosylated by T-Synthase,11 enabling LECs to potently activate CLEC-2 on the surface of platelets. The activated CLEC-2 dimer initiates a signaling cascade whereby the adaptor proteins Syk and Slp-76 activate phospholipase C-γ2, leading to platelet α-granule release and platelet aggregation on the LEC surface.9,10,16-18 The platelet aggregates that form as a result of podoplanin–CLEC-2 interaction make a platelet plug that physically blocks blood from entering the lower pressure lymphatic vessels.9,19
Lymphovenous hemostasis is functional both during lymphatic vessel development and in adult life, as postnatal loss of platelet CLEC-2 or podoplanin by inducible genetic deletion, antibody-mediated blocking, or bone marrow transplant confers the typical blood-filled lymphatic phenotype.8,20 Platelet aggregates are visible on LECs at the lymphovenous junction, the major site in the body where lymph drains from the thoracic duct into the blood circulation via the subclavian vein.8 At this site, specialized lymphovenous valves prevent the backflow of blood into the lymphatic circulation.21 The CLEC-2-podoplanin–dependent lymphovenous hemostasis mechanism acts as a second “fail-safe” mechanism to prevent movement of blood into the lymphatic system. Platelets also appear to have a second role in maintenance of barrier integrity of high endothelial venules in lymph nodes through interaction of platelet CLEC-2 with podoplanin expressed on fibroblastic reticular cells surrounding the high endothelial venule.20
Blood-lymphatic separation is critical for early lymph flow and lymphatic vessel maturation
Deficiency in either the lymphovenous valve or lymphovenous hemostasis results in severe lymphedema, chylothorax, and perinatal death.9,22 In CLEC-2–deficient mice, defective blood/lymphatic separation results in blood backflow into the lymphatic vessels, which impedes forward lymph flow, causing phenotypes indicative of lymphatic insufficiency despite the presence of a normal number of lymphatic vessels. Unexpectedly, loss of normal lymph flow has significant effects on the development and maturation of the lymphatic vasculature. CLEC-2–deficient mice exhibit a drastic reduction in lymphatic valve development due to a loss of flow-induced upregulation of several important lymphatic valve genes.23 A primary event in lymphatic valve development involves the shear stress-induced upregulation of GATA2 and FOXC2 in a subset of LECs located near a vessel bifurcation.24,25 These transcription factors, along with PROX1, coordinate a flow-induced gene expression program whereby Itga9,26 Calcineurin/NFATc1,27 Connexins 37 and 43,28 ephrinB2,29,30 Epsin,31 Nrp1,32 and likely others are expressed and mediate lymphatic valve development. The molecular interplay between GATA2, FOXC2, and PROX1 is not yet fully understood, but upregulation of all 3 transcription factors by flow is the only known mechanism underlying initiation of lymphatic valve development. Interestingly, FOXC2 expression is required for lymphatic valve maintenance as well as formation, as postnatal deletion of FOXC2 causes lymphatic valve regression and lymphatic insufficiency.33 Because platelets are required throughout life to maintain blood-lymphatic separation, postnatal disruption of lymphovenous hemostasis would disrupt lymph flow and possibly result in lymphatic valve regression and lymphedema. Given the abundance of antiplatelet therapies that are routinely prescribed to treat thrombotic and cardiovascular diseases, a better understanding of the effect of these antiplatelet therapies on lymphovenous hemostasis and lymphatic function is necessary. Heart failure induces high central venous pressure and increased lymph production; therefore, these patients require optimal lymphatic drainage to prevent edema. Future studies will determine the effect of antiplatelet therapy on lymphovenous hemostasis and whether these inhibitors have a deleterious effect on lymphatic function.
Secondary platelet responses in lymphovenous hemostasis
The discovery of lymphovenous clot formation raises important questions about how hemostatic processes are adapted to mediate this unique form of hemostasis. Unlike typical hemostatic events, clot formation at the lymphovenous junction arises independent of any vascular injury. Instead, platelets adhere and become activated on lymphatic endothelium, mediated by CLEC-2–podoplanin interaction.8 However, evidence suggests that CLEC-2 signaling alone is not sufficient to achieve lymphovenous hemostasis. Mice lacking β3-integrins, which mediate platelet-platelet interactions, showed deficient lymphovenous clot formation and an intermediate blood-lymph mixing phenotype with blood present in the terminal thoracic duct but not throughout the lymphatic system.8 This phenotype demonstrates the importance of secondary platelet aggregation in driving a stable plug to facilitate lymphovenous hemostasis.
Initiation of the lymphovenous clot, through podoplanin stimulation of platelet CLEC-2, induces tyrosine phosphorylation of the hemi-immunoreceptor tyrosine-based activation motif (ITAM) YxxL motif of the intracellular portion of CLEC-2, spurring subsequent platelet activation.10,16,34,35 Hemi-ITAM phosphorylation induces a cascade of phosphorylation events perpetuated through Syk, LAT, SLP-76, and PLCγ2, which culminates in cytosolic calcium concentrations rising, leading to platelet activation.36-39 The intracellular signaling cascade mirrors the other ITAM receptor-mediated activation pathway induced by glycoprotein 6 (GPVI) stimulation.35,39,40 Similarly to GPVI stimulation, CLEC-2 activation has been shown to be a potent platelet activating signal-driving αIIbβ3 integrin activation, both δ-granule and α-granule exocytosis, and adenosine 5′-diphosphate and thromboxane A2release.8,16,35,37,41,42 Thus, adherent platelets at the lymphovenous valve are likely capable of driving secondary activation after podoplanin stimulation. The precise contribution of secondary platelet responses in this process remains to be defined, but is clinically significant because these responses are the targets of commonly used antiplatelet drugs, such as aspirin and adenosine 5′-diphosphate receptor antagonists.
Mechanism of lymphovenous clot formation
Thrombin, a serine protease, is also a potent platelet agonist that drives platelet accumulation and fibrin deposition within clots.43-45 Fibrin deposition has been detected within lymphovenous clots formed in wild-type mice, suggesting that thrombin is generated locally.8 During blood vessel hemostasis, thrombin is typically generated at the site of vascular injury when tissue factor, constitutively expressed in the vessel adventitia, is exposed, initiating the extrinsic coagulation cascade.46,47 The lack of vascular damage during lymphovenous hemostasis suggests that vessel wall tissue factor is likely not exposed and raises the question about the source of procoagulant signaling within the clot. Possible intravascular sources of tissue factor at the lymphovenous junction include circulating blood cells and microparticles as well as activated endothelium.48-50
Tissue factor–independent coagulation may also be important in lymphovenous hemostasis. Platelet exposure of phosphatidylserine in response to activation is capable of initiating the intrinsic coagulation cascade.51 Procoagulant platelet activity is seen after potent activating signaling, typically involving Syk-mediated pathways including GPVI.46 Thus, during initial platelet recruitment to the lymphovenous LECs, CLEC-2 stimulation and platelet-derived soluble agonist production may drive procoagulant platelet activity, initiating thrombin formation and subsequent platelet activation and fibrin deposition.
Although thrombin activity and fibrin deposition occur during lymphovenous clot formation, it is not clear that coagulation is necessary to maintain lymphovenous hemostasis. In mouse models of various forms of hemophilia, no blood-filled lymphatic phenotypes have been reported.52 These mice may have an intermediate phenotype, similar to the β3 knockout mice, that is less obvious and escaped observation. However, lymphovenous hemostatic clots, which are small and function alongside valves, may simply not require thrombin activity to maintain blood/lymph separation.
Lymphovenous clot dynamics and the paradox of lymphovenous hemostasis
Proper function of the lymphovenous junction requires both the prevention of retrograde blood flow into the lymphatic system and the forward flow of lymph into the blood system. Thus, although clot formation prevents retrograde blood flow into lymphatics, it may, paradoxically, also prevent forward lymph flow. How lymphovenous clot formation and resolution at these multiple sites are coordinated with changes in lymphatic and venous pressures is an open and complex question that requires more research. Presently, it is assumed that lymphovenous clots are transient, but the timescale and mechanism of lymphovenous clot formation and resolution are unknown.
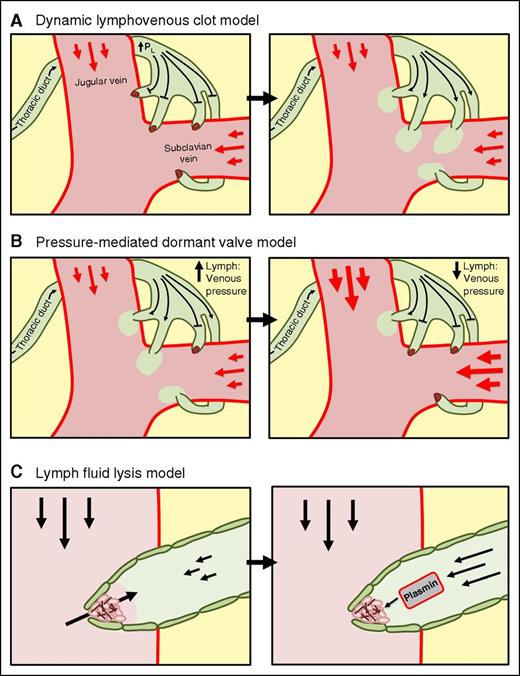
Figure 1 illustrates several possible mechanisms by which lymphovenous clot formation may simultaneously prevent retrograde blood flow and sustain forward lymph flow. One model is that lymphovenous clot formation occurs to prevent retrograde blood flow while transiently also blocking forward lymph flow, allowing lymphatic pressures to rise. When lymphatic pressure rises above a threshold, clots are dislodged and forward lymph flow is restored (Figure 1A). Alternatively, clot formation may occur only at a subset of lymphovenous junctions, rendering them temporarily dormant but allowing forward lymph flow to continue through the remaining active vessels (Figure 1B). Recent work has shown that the thoracic duct separates into smaller vessels at the lymphovenous junction, resulting in multiple lymphovenous junctions.21 In this model, the number of active/blocked lymphovenous junctions may act as a rheostat to adjust to changes in systemic lymphatic and venous pressures. In this model, clots would form at valves where retrograde blood flow occurs, while valves that maintain forward flow would remain open. A third model of lymphovenous clot dynamics relies on clot resolution mediated by the relative amount of blood or lymph fluid present at the lymphovenous junction. When venous pressures increase and drive blood into the lymphovenous junction, clotting occurs to prevent further retrograde blood flow. As lymphatic pressures rise, the clot is exposed to a lymph fluid environment, which is biochemically more thrombolytic than the blood,53 mediating clot resolution and allowing for forward lymph flow (Figure 1C).
Model of lymphovenous clot dynamics and lymph flow. (A) Lymphovenous clots block retrograde blood flow and increasing lymphatic pressures until the clots are resolved and a bolus of lymph fluid is infused into the bloodstream. (B) Lymphovenous clot formation at several lymphovenous junctions preventing retrograde or forward flow, but allowing flow in the remaining active vessels. (C) Lymphovenous clot formation occurs when the lymphovenous junction is in a blood environment, and the clot is resolved as lymph fluid envelops the clot, mediating fibrinolysis and forward lymph flow. PL, lymphatic pressure.
Model of lymphovenous clot dynamics and lymph flow. (A) Lymphovenous clots block retrograde blood flow and increasing lymphatic pressures until the clots are resolved and a bolus of lymph fluid is infused into the bloodstream. (B) Lymphovenous clot formation at several lymphovenous junctions preventing retrograde or forward flow, but allowing flow in the remaining active vessels. (C) Lymphovenous clot formation occurs when the lymphovenous junction is in a blood environment, and the clot is resolved as lymph fluid envelops the clot, mediating fibrinolysis and forward lymph flow. PL, lymphatic pressure.
Clinical implications of defective lymphovenous hemostasis
Various clinical conditions result in a failure of forward lymph flow at the lymphovenous junction and present with chylothorax and chylous ascites.54-57 Common causes of clinical lymphatic drainage defects include surgical trauma of the thoracic duct, lymphomas, heart failure, liver disease, and genetic lymphatic disorders.57 Both heart failure and liver disease patients show defective lymph flow as a result of increased venous pressures, causing obstruction at the lymphovenous junction.54,56-58 Ultrasound imaging of these patients reveals distended thoracic duct vessels, damaged lymphovenous valves, and retrograde blood flow.56,59 Interestingly, both heart failure60,61 and liver disease62,63 are also associated with hemostatic abnormalities. Perhaps under pathologically elevated venous pressures, lymphovenous clot formation becomes even more crucial to maintaining blood/lymph separation. In these pathological situations, if lymphovenous hemostasis is inhibited due to antiplatelet/anticoagulant treatments or pathological hemostatic defects, lymphatic insufficiency may be exacerbated, contributing to (lymph)edema. Importantly, it appears that very few CLEC-2–positive platelets are required for normal lymphovenous clot formation, as CLEC-2fl/fl; PF4-Cre mice have a milder phenotype than CLEC-2−/− mice, likely due to inefficient deletion of CLEC-2 in a small fraction of platelets.14 In addition, mixed bone marrow chimeras with relatively low fraction of wild-type to mutant platelets do not exhibit blood-lymphatic mixing.7 The need for only a small number of CLEC-2–positive platelets to achieve lymphovenous hemostasis explains why thrombocytopenic patients do not exhibit blood/lymphatic mixing and argues that lymphovenous hemostasis may only be disrupted in patients with both hemostatic deficiency and increased venous pressure. Further study of lymphovenous hemostasis in the disease setting will be crucial to determine the relationship of hemostatic pathways in shaping both lymphatic development and function under both normal and pathological conditions.
Authorship
Contribution: J.D.W., M.L.K., and D.T.S. contributed to writing and preparation of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel T. Sweet, University of Pennsylvania, Perelman School of Medicine, Translational Research Center, Room 11-123, 3400 Civic Center Blvd, Building 421, Philadelphia, PA 19104-5159; e-mail: dansweet@mail.med.upenn.edu.