Key Points
Pomalidomide is well tolerated in patients with AL amyloidosis; MTD of 4 mg/day on days 1 to 21 every 28 days.
Pomalidomide and dexamethasone can lead to HR of 50% in previously treated patients with AL amyloidosis.
Abstract
The objectives of a phase 1/2 trial of pomalidomide with dexamethasone for the treatment of light chain (AL) amyloidosis were to determine the safety, tolerability, maximum tolerated dose (MTD), recommended phase 2 dose, and hematologic and clinical response. A 3+3 dose-escalation phase (15 patients) was followed by an expansion cohort (12 patients) enrolled at the MTD. Pomalidomide was administered at 2 and 3 mg on days 1 to 28 (cohorts 1 and 2) and 4 mg on days 1 to 21 (cohort 3) every 28 days, with weekly dexamethasone at a dose of 20 mg. Twenty-seven patients with previously treated AL were enrolled, 15 during dose escalation (6 at 2 mg, 3 at 3 mg, and 6 at 4 mg) and 12 during dose expansion (all at 4 mg). One patient experienced dose-limiting toxicity at 4 mg; the MTD was determined as 4 mg. The most common grade ≥3 drug-related adverse events included myelosuppression and fatigue. Overall, hematologic response (HR) was 50% in 24 evaluable patients. The median time to best HR was 3 cycles, and median duration of HR was 15 months. Median overall survival has not yet been reached, with a median follow-up of 17.1 months and median event-free survival of 17.8 months. This trial was registered at www.clinicaltrials.gov as #NCT01570387.
Introduction
Immunomodulatory agents have efficacy in the treatment of light chain (AL) amyloidosis,1-5 either in combination with steroids and/or alkylating agents.6-8 In patients with AL amyloidosis, 15 mg/day lenalidomide is better tolerated than standard doses of 25 mg/day. Pomalidomide, a next-generation immunomodulatory agent, is effective in the treatment of patients with relapsed myeloma,9 including those who have previously failed lenalidomide.10
These findings prompted us to design a prospective phase 1/2 clinical trial of pomalidomide in the treatment of AL amyloidosis.
Study design
Eligibility criteria
This clinical trial was approved by the institutional review board in accordance with federal regulations and the Declaration of Helsinki. Eligibility included the following: a diagnosis of AL amyloidosis, a difference between involved and uninvolved serum free light chain levels ≥50 mg/L, ≥1 line of prior treatment, a platelet count ≥100 × 109/L, an absolute neutrophil count ≥1.5 × 109/L, a total bilirubin ≤1.5 mg/dL, aspartate aminotransferase/alanine aminotransferase ≤2 × upper limit of normal, a serum creatinine ≤3.0 mg/dL, and a performance status ≤2. All eligible patients were enrolled in the Risk Evaluation and Mitigation Strategies program.
Treatment
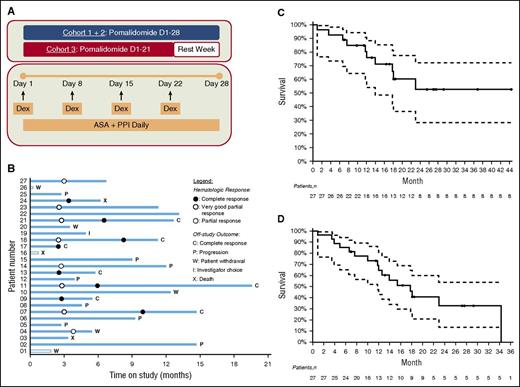
The study consisted of a dose-escalation phase to determine the maximum tolerated dose (MTD), followed by an expansion cohort treated at the established MTD. Patients received oral pomalidomide on days 1 to 28 of a 28-day cycle for cohort 1 (2 mg) and cohort 2 (3 mg) and days 1 to 21 of a 28-day cycle for cohort 3 (4 mg). Dexamethasone was administered at 20 mg weekly. All patients were required to take a daily full-dose aspirin for prevention of thromboembolic complications (Figure 1A).
Phase I/II trial of pomalidomide and dexamethasone in AL amyloidosis. (A) Treatment schema. (B) Outcome analysis: each bar represents an individual patient. The abscissa represents the time on study. (C) Overall survival from enrollment by Kaplan-Meier analysis. (D) Event-free survival by Kaplan-Meier analysis.
Phase I/II trial of pomalidomide and dexamethasone in AL amyloidosis. (A) Treatment schema. (B) Outcome analysis: each bar represents an individual patient. The abscissa represents the time on study. (C) Overall survival from enrollment by Kaplan-Meier analysis. (D) Event-free survival by Kaplan-Meier analysis.
Dose escalation followed a standard 3+3 design, based on dose-limiting toxicities (DLTs) experienced during cycle 1. Three patients were initially enrolled to cohort 1. If none of these patients experienced a DLT, 3 patients were to be enrolled to cohort 2. However, if 1 of the first 3 patients experienced a DLT, 3 additional patients were enrolled to that cohort; if none of these additional patients experienced a DLT, dose escalation continued. The MTD was defined as the highest dose of pomalidomide resulting in a DLT during cycle 1 in ≤1 of 6 patients. DLTs were defined as the following: grade 4 thrombocytopenia or neutropenia lasting ≥7 days, grade 3 thrombocytopenia with significant bleeding, grade 3 febrile neutropenia with infection, or any grade ≥3 nonhematologic toxicity. Treatment was continued until disease progression, development of toxicity, or achievement of a complete hematologic response (CR).
Objectives
The primary objective was to establish the MTD of pomalidomide in subjects with previously treated AL amyloidosis. Secondary objectives included hematologic response, duration of hematologic response, organ response, time to event, and overall survival. An additional exploratory objective was to investigate the changes in B-type natriuretic peptide (BNP) while on pomalidomide.
Toxicity and response
The National Cancer Institute, Common Terminology Criteria for Adverse Events, version 4.03, was used to grade adverse events (AEs). Toxicity was defined as an AE if it was considered to be possibly, probably, or definitely related to treatment. The hematologic and organ response criteria were defined by consensus opinion.11,12 Responses were assessed after the completion of 3 cycles and every 3 cycles thereafter.
Statistical considerations
The safety population included all patients who received ≥1 dose of pomalidomide. The efficacy-evaluable population comprised of patients who received ≥3 cycles and had evaluable response assessments. Response was assessed in the phase 1 and phase 2 groups separately. Time to hematologic progression (defined from treatment initiation), time to subsequent therapy (defined as the time to first alternative treatment), and overall survival were evaluated. Kaplan-Meier methodology was used for time-to-event analyses. All efficacy analyses were descriptive without statistical comparisons between dose groups.
Results and discussion
From 2012 to 2015, 27 patients with AL amyloidosis were enrolled, 15 in phase 1 and 12 in the expansion phase 2 cohort. Baseline patient characteristics are listed in Table 1.
Patient characteristics
| . | No. of patients* . |
|---|---|
| Median age, years (range) | 68 (44-79) |
| Male/female | 16/11 |
| κ/λ isotype | 9/18 |
| ≥2 organs involved, n (%) | 17 (63.0%) |
| Renal involvement, n (%) | 14 (51.8%) |
| Median proteinuria, mg/24 h (range) | 553 (0-14 141) |
| Cardiac involvement, n (%) | 18 (66.7%) |
| Biomarker stage II and III | 23 (85.2%) |
| Median and mean BNP, pg/mL | 174 and 252 |
| Prior treatment, median (range) | 2 (1-6) |
| Prior treatment with HDM/SCT, n (%) | 16 (59.3%) |
| Prior treatment with bortezomib, n (%) | 21 (77.8%) |
| Prior treatment with lenalidomide, n (%) | 13 (48.1%) |
| Time to enrollment, median months (range) | |
| From diagnosis | 27 (4-246) |
| From last treatment | 5 (1-89) |
| . | No. of patients* . |
|---|---|
| Median age, years (range) | 68 (44-79) |
| Male/female | 16/11 |
| κ/λ isotype | 9/18 |
| ≥2 organs involved, n (%) | 17 (63.0%) |
| Renal involvement, n (%) | 14 (51.8%) |
| Median proteinuria, mg/24 h (range) | 553 (0-14 141) |
| Cardiac involvement, n (%) | 18 (66.7%) |
| Biomarker stage II and III | 23 (85.2%) |
| Median and mean BNP, pg/mL | 174 and 252 |
| Prior treatment, median (range) | 2 (1-6) |
| Prior treatment with HDM/SCT, n (%) | 16 (59.3%) |
| Prior treatment with bortezomib, n (%) | 21 (77.8%) |
| Prior treatment with lenalidomide, n (%) | 13 (48.1%) |
| Time to enrollment, median months (range) | |
| From diagnosis | 27 (4-246) |
| From last treatment | 5 (1-89) |
HDM/SCT, high-dose melphalan and autologous stem cell transplantation.
27 patients total were enrolled in the study.
DLTs and MTD
The MTD of pomalidomide was established at 4 mg when 1 DLT (grade 3 pneumonia with neutropenia) was observed in a patient in cohort 3 (4 mg). Of note, 3 additional patients were enrolled in this cohort without any additional DLTs. Cohort 1 was also expanded to enroll a total of 6 patients due to grade 3 renal failure in cycle 3 (not considered DLT) in 1 of the first 3 patients.
Treatment experience
Treatment outcomes are summarized in Figure 1B. Of the 27 patients, 24 completed ≥3 cycles of therapy, and the median number of cycles administered was 6 (range, 0-18). Twenty-four patients discontinued treatment due to disease progression (n = 8), patient choice (n = 5), CR (n = 7), investigator choice (n = 1), or death (n = 3). No patient discontinued treatment due to excessive toxicity.
Deaths
Ten patients have died. There were 3 deaths while on study, 2 of which were related to infection: 1 in cohort 1 due to Klebsiella pneumoniae and 1 in the expansion cohort due to Legionella pneumonia. The third death occurred >30 days after discontinuation of the study drug. None of these deaths were associated with neutropenia or thrombocytopenia.
Toxicities
Treatment-related AEs are summarized in Table 2. Grade 3 and 4 myelosuppression occurred in 7 patients (25.9%), which improved by holding or reducing the pomalidomide dose. Severe fatigue occurred in 5 patients (18.5%). Skin rash was mild and occurred in 9 patients (33.3%). Upper respiratory infections occurred in 8 (29.6%) patients, none of whom were neutropenic. Grade 3 pneumonia occurred in 3 (11.1%) patients; only 1 was associated with neutropenia. Seven patients (25.9%) had worsening renal function, of which 2 (7.4%) had a grade 3 to 4 event. No patient required dialysis. No thromboembolic complications were observed.
Treatment-related AEs
| AE . | Grades 1 and 2, No. (%) . | Grades 3 and 4, No. (%) . |
|---|---|---|
| Fatigue | 12 (44.4%) | 5 (18.5%) |
| Skin rash | 9 (33.3%) | 0 (0%) |
| Upper respiratory infection | 6 (22.2%) | 2 (7.4%) |
| Pneumonia | 0 (0%) | 3 (11.1%) |
| Increased creatinine | 5 (18.5%) | 2 (7.4%) |
| Myelosuppression | 16 (59.3%) | 7 (25.9%) |
| Thromboembolic events | 0 (0%) | 0 (0%) |
| AE . | Grades 1 and 2, No. (%) . | Grades 3 and 4, No. (%) . |
|---|---|---|
| Fatigue | 12 (44.4%) | 5 (18.5%) |
| Skin rash | 9 (33.3%) | 0 (0%) |
| Upper respiratory infection | 6 (22.2%) | 2 (7.4%) |
| Pneumonia | 0 (0%) | 3 (11.1%) |
| Increased creatinine | 5 (18.5%) | 2 (7.4%) |
| Myelosuppression | 16 (59.3%) | 7 (25.9%) |
| Thromboembolic events | 0 (0%) | 0 (0%) |
Responses
Hematologic responses are summarized in Table 3. The overall hematologic response rate was 50.0% (12 of 24). The response rate by intention-to-treat was 44.4% (12 of 27). The median time to best hematologic response was 3 cycles (range, 3-9). These response rates include CRs at 3 mg in 2 patients and at 4 mg in 2 patients in phase 1. Median duration of CR was 15 months (range, 3-26). Three of the 13 patients (23.1%) who had been previously treated with lenalidomide and had subsequently progressed achieved a CR.
Hematologic response to therapy
| . | Phase 1 . | Phase 2 . | Total . |
|---|---|---|---|
| Evaluable patients* | 14 | 10 | 24 |
| CR | 4 | 4 | 8 |
| VGPR | 0 | 1 | 1 |
| PR | 2 | 1 | 3 |
| Response ≥ VGPR | 4 | 5 | 9 (37.5%) |
| . | Phase 1 . | Phase 2 . | Total . |
|---|---|---|---|
| Evaluable patients* | 14 | 10 | 24 |
| CR | 4 | 4 | 8 |
| VGPR | 0 | 1 | 1 |
| PR | 2 | 1 | 3 |
| Response ≥ VGPR | 4 | 5 | 9 (37.5%) |
PR, partial response; VGPR, very good partial response.
Evaluable patients: completing 3 cycles of treatment.
There was a paradoxical increase of BNP by 30% in 24 patients (88.8%) after 3 cycles of therapy. The mean BNP increased from 252 to 695 pg/mL after 3 cycles. This was not associated with any clinical worsening of congestive heart failure. Renal response occurred in 7.1% (1 of 14) of the patients with renal involvement at 12 months.
Survival
The median overall survival for 27 patients has not been reached with a median follow-up of 17.1 months (range, 0.9-44.3 months). The median event-free survival is 17.8 months (95% confidence interval: 11.8, not attained; Figure 1C-D).
Discussion
The MTD of pomalidomide was similar to the dosing established in myeloma. This is in contrast to lenalidomide, which is not tolerated in patients with AL amyloidosis at the standard dose used in myeloma.3,5 Hematologic CR, time to hematologic response, and duration of response achieved by pomalidomide/dexamethasone were favorable compared with lenalidomide in AL amyloidosis.13 Treatment-related adverse events of skin rash, myelosuppression, and worsening of serum creatinine were less common than reported with lenalidomide in a previous trial.3 Organ response rate was relatively low; however, this was not different from the 0% to 26% reported with other regimens in relapsed AL patients.3,5,8 The low organ response rate may be due in part to a short follow-up time. Most notably, hematologic responses to pomalidomide were seen even in patients who had prior lenalidomide.
Although pomalidomide/dexamethasone has already been shown to have activity in AL amyloidosis by Dispenzieri et al,14 our trial is different in that it includes a dose-escalation format to arrive at an MTD. This study design was prompted by the experience of lenalidomide dosing in AL amyloidosis.
A summary of the results of this study was presented at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 7, 2014, and the XV International Symposium on Amyloidosis, Uppsala, Sweden, July 6, 2016.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the numerous colleagues in the Amyloidosis Center, Cancer Clinical Trials Office, and Center for Cancer and Blood Disorders at Boston Medical Center who assisted with the multidisciplinary evaluation and treatment of the patients with AL amyloidosis. The authors also thank Dr Fangui Sun for performing statistical analysis.
This trial was partly supported by grants from the Celgene Corporation.
Authorship
Contribution: V.S. designed research, collected and analyzed data, and wrote the manuscript; A.C.S. collected data and performed research; S.L. collected and analyzed data; C.V. edited the manuscript; J.M.S. edited the manuscript; D.C.S. designed research and collected and analyzed data; and all authors approve of the manuscript and data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
David C. Seldin died on June 27, 2015.
Correspondence: Vaishali Sanchorawala, Section of Hematology/Oncology, FGH 1007, 820 Harrison Ave, Boston, MA 02118; e-mail: vaishali.sanchorawala@bmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal