Key Points
Anoxia combined with glucose supplementation maintains viability of neutrophils for 20 hours without affecting their functions.
Such conditioned neutrophils are suitable for efficient DNA transfection and transfusion.
Abstract
Functional studies of human neutrophils and their transfusion for clinical purposes have been hampered by their short life span after isolation. Here, we demonstrate that neutrophil viability is maintained for 20 hours in culture media at 37°C under anoxic conditions with 3 mM glucose and 32 μg/mL dimethyloxalylglycine supplementation, as evidenced by stabilization of Mcl-1, proliferating cell nuclear antigen (PCNA), and pro-caspase-3. Notably, neutrophil morphology (nucleus shape and cell-surface markers) and functions (phagocytosis, degranulation, calcium release, chemotaxis, and reactive oxygen species production) were comparable to blood circulating neutrophils. The observed extension in neutrophil viability was reversed upon exposure to oxygen. Extending neutrophil life span allowed efficient transfection of plasmids (40% transfection efficiency) and short interfering RNA (interleukin-8, PCNA, and Bax), as a validation of effective and functional genetic manipulation of neutrophils both in vitro and in vivo. In vivo, transfusion of conditioned neutrophils in a neutropenic guinea pig model increased bacterial clearance of Shigella flexneri upon colonic infection, strongly suggesting that these conditioned neutrophils might be suitable for transfusion purposes. In summary, such conditioning of neutrophils in vitro should facilitate their study and offer new opportunities for genetic manipulation and therapeutic use.
Introduction
Human polymorphonuclear neutrophils (neutrophils) are commonly described as “short-lived cells,” as compared with lymphocytes, mast cells, or other phagocytic cells such as macrophages. Neutrophil life span was measured upon neutrophil labeling ex vivo prior to transfusion in vivo and was estimated to be between 1.5 and 10 hours in mice and humans.1 A recent study based on oral administration of deuterated water concluded that human circulating neutrophil life span could reach 5.4 days2 ; however, this appealing result remains discussed by other groups.3
In vitro, it is well known that purified neutrophil life span is limited, barely exceeding 8 hours under atmospheric conditions containing 21% O2 (pO2 = 160 mmHg). Consequently, these cells remain difficult to culture and manipulate; indeed, neutrophils stand as the last white blood cell population that cannot be efficiently manipulated genetically despite previous attempts.4,5 In addition, neutrophil transfusion efficiency has not been demonstrated despite its urgent need in many life-threatening multiresistant bacterial or fungal infections in neutropenic patients.6,7 It is well known that neutrophil transfusion efficiency relies on dose and viability of the transfused cells.8,9 In conclusion, increasing neutrophil viability in vitro represents a major challenge to allow neutrophil physiology and antimicrobial function studies but also to promote their clinical use.
Walmsley et al10 have previously shown that the survival of neutrophils was extended in low oxygen environments and that this beneficial effect, mediated at least in part by HIF-1α, was higher under anoxia (pO2 = 0 mmHg) than hypoxia (pO2 = 3 kPa or 22.5 mmHg). HIF-1α subunit abundance decreases under normoxic conditions because of hydroxylation by prolyl hydroxylases and subsequent targeting to proteasomal degradation.11,12 Prolyl hydroxylase 3 was shown to be essential for HIF-1α degradation in neutrophils13 and was inhibited by the pan-hydroxylase inhibitor dimethyloxalylglycine (DMOG), which decreased neutrophil apoptosis.13 Importantly, the authors demonstrated that neutrophils were transcriptionally active (g3pdh, mif, nfκb) under these conditions.10
Here, we demonstrate that neutrophil life span is increased in vitro when conditioned for 20 hours at 37°C in anoxic culture medium supplemented with 3 mM glucose and 32 μg/mL DMOG. Conversely, neutrophils exposed 20 hours at 37°C under atmospheric conditions (+O2) in RPMI medium only are named unconditioned neutrophils. Conditioned neutrophils remained viable, and we show that their morphology, degranulation, reactive oxygen species (ROS) production, chemotaxis, and bactericidal properties are similar to freshly purified neutrophils. Furthermore, we demonstrate that conditioning neutrophils in these conditions allows efficient DNA transfection in vitro and efficient transfusion in vivo.
Materials and methods
Human blood collection
All participants gave written informed consent in accordance with the Declaration of Helsinki principles. Peripheral human blood was collected from healthy patients at the Investigation Clinique et Accès aux Ressources Biologiques service of the Pasteur Institut (authorization DC No.2008-68) and from healthy patients stimulated 5 days with granulocyte colony-stimulating factor (G-CSF) at the Gustave Roussy Cancer Campus (Villejuif, France). Human blood was collected from the antecubital vein into tubes containing sodium citrate (3.8% final) as anticoagulant molecules. When specified, sodium heparinate (1% final concentration) was alternatively used.
Neutrophil isolation
Neutrophils were isolated from total blood samples immediately after blood collection with citrate or heparin as anticoagulant molecules.
Blood samples (50 mL) were centrifuged at 450g for 15 minutes. Platelet-rich plasma was collected and centrifuged at 2500g for 20 minutes to form platelet-poor plasma (PPP). Collected blood cells were resuspended in NaCl 0.9% and dextran sulfate (0.72%). After 30 minutes sedimentation, the upper layer, containing neutrophils, was centrifuged at 240g for 10 minutes. The pellet was resuspended in 1 mL of PPP, and cells were then separated on a Percoll (GE Healthcare) gradient (lower phase, 51% Percoll/49% PPP; upper phase, 42% Percoll/58% PPP) by centrifugation at 240g for 20 minutes. Neutrophils were collected and washed in RPMI medium 1640 (Sigma-Aldrich).
In order to increase the purity of the neutrophil-enriched fraction, remaining dead cells were removed using Dead Cell Removal MicroBead (negative selection; Miltenyi Biotec, Auburn, CA), and red blood cells were removed using CD235a (glycophorin) microbeads (negative selection; Miltenyi Biotec).
Neutrophil conditioning medium
Neutrophil fractions with >95% purity were resuspended at 5 × 106 cells/mL in RPMI 1640 with 2 mM l-glutamine, 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES; Gibco, Life Technologies), 10% heat-inactivated fetal bovine serum (Invitrogen, Gibco), the indicated concentrations of glucose (Sigma-Aldrich), and DMOG (Cayman Chemical Company).
For comparative experiments aimed at characterizing the role of oxygen on neutrophil viability, purified neutrophils were split into 2 identical fractions: one that was exposed to atmospheric conditions at 37°C with 5% CO2 (21% O2 condition, +O2) and a second one that was conditioned at 37°C in an anoxic chamber (Minimacs, Don Withley) under an atmosphere containing 90% N2, 5% CO2, and 5% H2 (0% O2 condition, −O2), during the indicated time. H2 is used by a palladium catalyzer to remove oxygen traces in the chamber.
The optimal storage condition described in this study, 20 hours postpurification was obtained under anoxic conditions in RMPI supplemented with 3 mM glucose and 32 μg/mL DMOG. In this medium, the absolute cell count was assessed using a Malassez cell, and the neutrophil survival rate was determined by flow cytometry.
Flow cytometry
Cell viability.
For neutrophil viability analysis, human neutrophils (106 cells) were washed twice in phosphate-buffered saline and resuspended in 1 mL of Annexin V binding buffer; 100 µL sample (1 × 105 cells) was stained with 5 µL of allophycocyanin–Annexin V and 5 µL of propidium iodide (PI; 0.5 µg/mL final concentration). At least 104 events were acquired for each condition on a FACS-Calibur flow cytometer (BD Biosciences), and data were analyzed using CellQuest Pro Software (BD Biosciences). Viable cells were defined through Annexin V negative (Annexin V−)/PI negative (PI−) quantifications.
Quantification of neutrophil cell-surface markers.
Neutrophils (2 × 106) purified with citrate were infected with S. flexneri at a multiplicity of infection (MOI) 20 in RMPI + HEPES (10 mM). After 10 minutes centrifugation at 300g, infected neutrophils were incubated during 30 minutes at 37°C. Cell-surface markers exposure was quantified by flow cytometry as follows: 2.5 × 105 purified cells or 1 × 109 whole blood cells were collected and incubated with conjugated antibodies (see supplemental Methods, available on the Blood Web site). Cell labeling was analyzed using a FACS-Calibur flow cytometer (BD Biosciences), and data were analyzed using CellQuest Pro Software (BD Biosciences). Results are representative of 3 independent experiments.
Plasmid or siRNA nucleofection
Plasmid or short interfering RNA (siRNA) transfections were performed by nucleofection (Lonza) on neutrophils purified with citrate (2 × 106 cells per transfection). Neutrophils were preincubated 2 hours in 500 µL of supplemented culture media (RPMI + 10% fetal calf serum + 3 mM glucose + 32 µg/mL DMOG) at 37°C under anoxic conditions. Cells were collected by centrifugation (300g, 10 minutes at room temperature) and washed in RPMI. After centrifugation, the pellets were suspended in 100 µL supplemented Mouse T Cell Nucleofector solution (Lonza); 2 µg of pmaxGFP plasmid (Lonza), 1 μM interleukin-8 (IL-8) siRNA (SMARTpool: Accell IL8 siRNA; Thermo Fischer Scientific), proliferating cell nuclear antigen (PCNA) siRNA (Applied Biosystems), Bax siRNA (Santa Cruz), or negative control siRNA (Qiagen) were added before transferring the mixture into nucleofection cuvettes. DNA transfer was performed by electroporation using Nucleofector Program Y-01 (Amaxa Nucleofactor, Lonza). Transfected cells were analyzed 20 hours postnucleofection by flow cytometry, fluorescence imaging (plasmid), quantitative polymerase chain reaction, enzyme-linked immunosorbent assay, or western blot (siRNA) (supplemental Methods).
Neutrophil transfusion in guinea pig
Neutrophil transfusions were performed in neutropenic Dunkin-Hartley guinea pigs (150 g). Neutropenia was generated by 2 intraperitoneal injections of cyclophosphamide (100 mg/kg on day 7 and 50 mg/kg on day 1). When indicated, guinea pigs were infected intrarectally with S. flexneri (109 colony-forming unit [CFUs]). When indicated, neutrophils purified from guinea pig or human were transfused (5 × 106 or 107, respectively), either freshly purified, conditioned for 20 hours, or not conditioned, to neutropenic guinea pigs by intracardiac injection 1 hour postinfection. After conditioning and prior transfusion, neutrophils are kept away from atmospheric exposure to avoid reoxygenation. Neutrophil transfusion efficiency was assessed 8 hours postinfection by quantifying colonic S. flexneri CFU and myeloperoxidase (MPO) activity (supplemental Methods). Experiments were performed on 3 animals per condition.
Transfused human neutrophil recovery was calculated by transfusing 107 human neutrophils (freshly purified, conditioned, or transfected) in naïve neutropenic guinea pigs. Eight hours posttransfusion, human neutrophils were purified from 2 mL citrated blood and labeled with CD66b-PE and PI; viable (PI−) neutrophils were counted by flow cytometry using Trucount tubes (BD Biosciences). The percentage of CD15+/PI− recovery rate was calculated using 6 mL total blood volume. Experiments were performed on 4 animals for each condition.
Statistical analysis
Data were analyzed using Prism 5.0 software (GraphPad) using Student t test. Significance was accepted when P < .05.
Results
Neutrophil viability is maintained under anoxia
Neutrophil viability was evaluated by flow cytometry, by quantifying the proportion of viable (PI−) and nonapoptotic (Annexin V−) cells. Neutrophils purified from citrated blood were separated from remaining red blood cells with CD235a Microbeads (supplemental Figure 1). We showed that neutrophil viability at 37°C in RPMI medium was significantly increased under anoxia 20 hours or 48 hours postpurification, compared with atmospheric conditions (supplemental Figure 2A). In the absence of oxygen, cellular energy production relies on anaerobic glycolysis, using glucose as a substrate.14,15 Accordingly, neutrophil viability was further increased 20 hours and 48 hours postpurification by supplementing the anoxic culture medium with 3 mM glucose, whereas no beneficial effect of glucose supplementation was observed under atmospheric conditions (supplemental Figure 2A).
Walmsley et al previously demonstrated that neutrophil apoptosis was reduced when stabilizing HIF-1α in the presence of DMOG.10 Accordingly, in a glucose-supplemented anoxic culture medium, the presence of DMOG (32 μg/mL) significantly increased neutrophil viability compared with atmospheric conditions without supplementation (supplemental Figure 2B). Under atmospheric conditions, a beneficial effect of DMOG was observed to a lower extent, consistent with the antiapoptotic role of HIF-1α previously described.10 When heparinized blood was used instead of citrated blood, neutrophil viability was also maintained 20 hours and 48 hours postpurification under anoxic conditions, in the presence of glucose and DMOG, however to a lower extent (supplemental Figure 3A-C). This might be because of higher levels of neutrophil activation generated by heparin compared with citrate.16
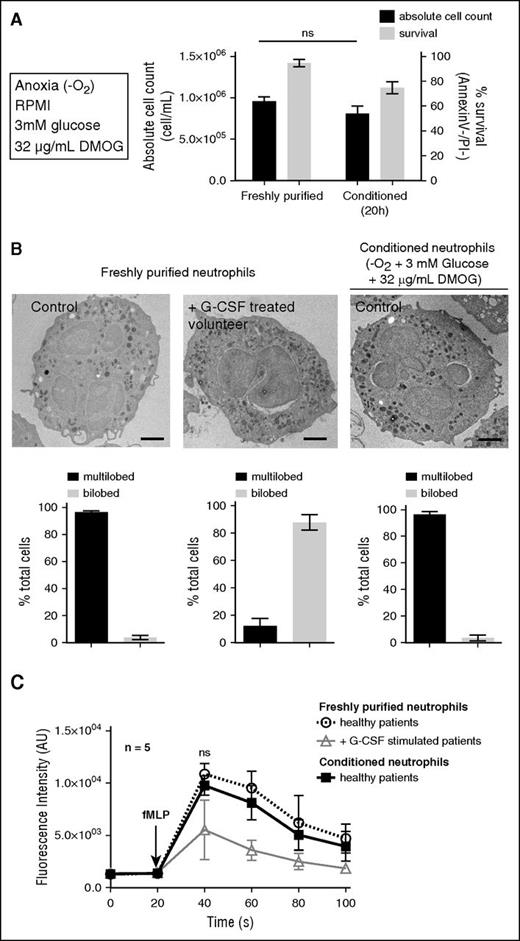
In summary, the optimal conditions for neutrophil viability were anoxic conditions, at 37°C in RPMI containing 10 mM HEPES, supplemented with 3 mM glucose and 32 mg/mL DMOG (Figure 1A). After 20 hours conditioning in this medium, the absolute neutrophil count was unchanged, and the survival rate reached 74 ± 2% (P < .05) 20 hours postpurification (vs 38 ± 14% under atmospheric conditions without supplementation (Figure 1A). Prior to analysis or functional characterization, viable conditioned neutrophils (hereafter named “conditioned neutrophils”) were purified by negative selection to obtain a pure Annexin V−/PI− population (supplemental Figure 4).
Human neutrophil survival is extended while conditioned under anoxic conditions. (A) Neutrophil viability (Annexin V−/PI−) and absolute cell count were calculated on freshly purified neutrophils or after 20 hours incubation under anoxic conditions at 37°C in RPMI + HEPES (10 mM) with 3 mM glucose and 32 μg/mL DMOG supplementation. Error bars indicate standard deviation (SD). “ns” indicates P > .05 (n = 5). (B) Freshly purified and conditioned neutrophil morphology was assessed by transmission electron microscopy and compared with G-CSF–stimulated volunteer neutrophils (pseudo-Pelger-Huët phenotype). Bars represent 1 μm. The proportions of multilobed or bilobed neutrophils were calculated for each condition. (C) The cytosolic calcium flux was assessed on neutrophils described in panel B upon stimulation with fMLF (10 μM) in RPMI medium at 37°C, every 20 seconds. Error bars indicate SD. “ns” indicates P > .05 (n = 5).
Human neutrophil survival is extended while conditioned under anoxic conditions. (A) Neutrophil viability (Annexin V−/PI−) and absolute cell count were calculated on freshly purified neutrophils or after 20 hours incubation under anoxic conditions at 37°C in RPMI + HEPES (10 mM) with 3 mM glucose and 32 μg/mL DMOG supplementation. Error bars indicate standard deviation (SD). “ns” indicates P > .05 (n = 5). (B) Freshly purified and conditioned neutrophil morphology was assessed by transmission electron microscopy and compared with G-CSF–stimulated volunteer neutrophils (pseudo-Pelger-Huët phenotype). Bars represent 1 μm. The proportions of multilobed or bilobed neutrophils were calculated for each condition. (C) The cytosolic calcium flux was assessed on neutrophils described in panel B upon stimulation with fMLF (10 μM) in RPMI medium at 37°C, every 20 seconds. Error bars indicate SD. “ns” indicates P > .05 (n = 5).
Conditioned neutrophil morphology and abundance of cell-surface markers
The morphology of freshly purified or conditioned neutrophils was analyzed by electron microscopy. No difference was observed regarding the nucleus multilobular shape or the granular content (Figure 1B). G-CSF was previously shown to prolong neutrophil survival,17,18 in particular by inhibiting the increase in intracellular calcium levels associated with apoptosis.19,20 It was also described that G-CSF induced pseudo-Pelger-Huët anomalies, similar to the ones observed in hereditary neutrophils morphological modifications.21 Consistently, symmetric bilobed nuclei in neutrophils purified from G-CSF–stimulated volunteers were observed (Figure 1B), which is a typical pseudo-Pelger-Huët anomaly. No comparable anomaly was observed in freshly purified or conditioned neutrophils (Figure 1B). In addition, upon N-formyl-methionyl-leucyl-phenylalanine (fMLF) stimulation, the increase in intracellular calcium concentration in freshly purified neutrophils was comparable to the one in conditioned neutrophils, whereas it was reduced in neutrophils from G-CSF–stimulated volunteers (Figure 1C), as previously reported20 (G-CSF treatment leading to increased concentrations of banded neutrophils and progenitors with altered reactivity for fMLF).
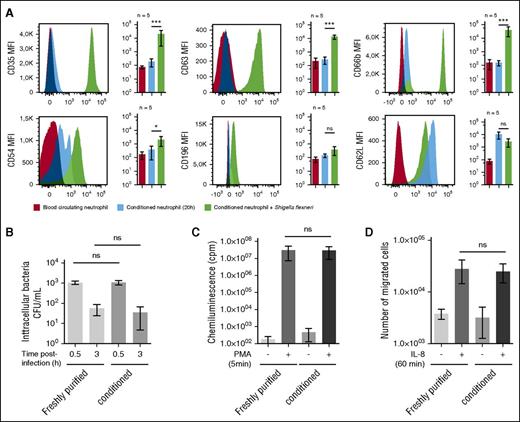
The abundance of physiological cell-surface markers such as the neutrophil-specific epitope CD6622 (data not shown), CD63 (primary granules), CD66b (secondary granules), the C3b receptor (CD35),2,8 the chemokine receptor CCR6 (CD196),23 the adhesion molecule Icam-1 (CD54),24 or the l-selectin CD62L were not significantly different on whole blood or conditioned neutrophils (Figure 2A). Whereas conditioned neutrophil cell-surface abundance of CD35, CD63, CD66b, and CD54 was significantly increased upon S. flexneri infection (Figure 2A). Moreover, the l-selectin CD62L level was increased in conditioned neutrophils as compared with whole blood neutrophils, and its level was reduced upon S. flexneri infection (Figure 2A).
Conditioned neutrophil stimulation and antibacterial functions are similar to blood circulating neutrophils. (A) Neutrophil cell-surface markers were quantified on circulating and conditioned neutrophils, either naïve or infected by S. flexneri (MOI 20, 30 minutes). The following cell-surface markers were quantified by flow cytometry: CD35 (C3bR), CD63 (primary granule), CD66b (secondary granules), CD54 (ICAM-1), CD196 (CCR6), and CD62L (l-selectin). Results are expressed as a mean of fluorescence intensity (MFI) calculated on the whole neutrophil population. “ns” indicates P > .05; *P < .05; ***P < .001 (n = 5). (B) S. flexneri phagocytosis and killing efficiencies were assessed with freshly purified or conditioned (20 hours) neutrophils, at MOI 20 during respectively 30 minutes (phagocytosis) and 180 minutes (killing) at 37°C. Intracellular bacteria were counted by plating after gentamycin treatment. Error bars indicate SD. “ns” indicates P > .05 (n = 3). (C) PMA-induced ROS production was assessed by chemoluminescence changes (cpm) on freshly purified and conditioned neutrophils incubated with luminol (10 μM) and stimulated with PMA (200 ng/ml) for 5 minutes. “ns” indicates P > .05 (n = 5). (D) Freshly purified and conditioned neutrophil chemotaxis properties were assessed using Transwells. Neutrophils (2 × 105) were loaded in the upper chamber, and IL-8 (20 nM) was added in the lower buffer. Migrated neutrophils were counted in the lower chamber after 1 hour incubation at 37°C. “ns” indicates P > .05 (n = 5).
Conditioned neutrophil stimulation and antibacterial functions are similar to blood circulating neutrophils. (A) Neutrophil cell-surface markers were quantified on circulating and conditioned neutrophils, either naïve or infected by S. flexneri (MOI 20, 30 minutes). The following cell-surface markers were quantified by flow cytometry: CD35 (C3bR), CD63 (primary granule), CD66b (secondary granules), CD54 (ICAM-1), CD196 (CCR6), and CD62L (l-selectin). Results are expressed as a mean of fluorescence intensity (MFI) calculated on the whole neutrophil population. “ns” indicates P > .05; *P < .05; ***P < .001 (n = 5). (B) S. flexneri phagocytosis and killing efficiencies were assessed with freshly purified or conditioned (20 hours) neutrophils, at MOI 20 during respectively 30 minutes (phagocytosis) and 180 minutes (killing) at 37°C. Intracellular bacteria were counted by plating after gentamycin treatment. Error bars indicate SD. “ns” indicates P > .05 (n = 3). (C) PMA-induced ROS production was assessed by chemoluminescence changes (cpm) on freshly purified and conditioned neutrophils incubated with luminol (10 μM) and stimulated with PMA (200 ng/ml) for 5 minutes. “ns” indicates P > .05 (n = 5). (D) Freshly purified and conditioned neutrophil chemotaxis properties were assessed using Transwells. Neutrophils (2 × 105) were loaded in the upper chamber, and IL-8 (20 nM) was added in the lower buffer. Migrated neutrophils were counted in the lower chamber after 1 hour incubation at 37°C. “ns” indicates P > .05 (n = 5).
Bactericidal properties of conditioned neutrophils are similar to freshly purified neutrophils
We demonstrated that S. flexneri was efficiently phagocytized and killed, as previously reported25 (Figure 2B). The PMA-induced ROS production (Figure 2C) and the IL-8-dependent chemotaxis (Figure 2D) properties of conditioned neutrophils were not significantly different from freshly purified neutrophils, further confirming that the conditioning process does not alter neutrophil bactericidal functions and motility.
Conditioned neutrophils are efficiently transfected
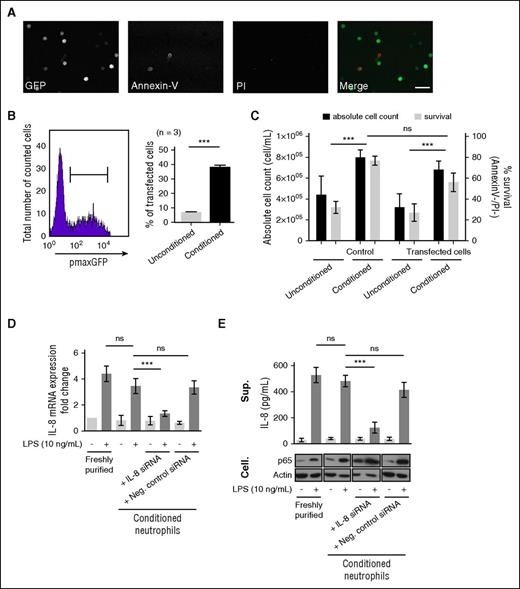
The extended viability of conditioned neutrophils was hypothesized to allow an increase in DNA transfer efficiency. Indeed, so far, plasmid transfer in neutrophils by nucleofection was inefficient 2 hours posttransfection (∼5%).4,5 The extended viability of conditioned neutrophils allowed longer transfection experiments, up to 20 hours. We demonstrated that conditioned neutrophils were efficiently transfected with pmaxGFP plasmid (39 ± 4% of total cells) while analyzed 20 hours posttransfection (Figure 3A-B); without altering neutrophil viability, only 10% of transfected cells were PI+ upon nucleofection (supplemental Figure 5), consistent with previous reports.4,5 The absolute cell count was unchanged upon transfection (Figure 3C). Conditioned neutrophils were efficiently transfected with IL-8-siRNA by nucleofection in lipopolysaccharide (LPS)-stimulated neutrophils26,27 (Figure 3D). IL-8 expression was significantly reduced in neutrophils transfected with IL-8 siRNA, 20 hours postnucleofection, but not in negative control siRNA-transfected cells (Figure 3D). These results were further confirmed by quantifying the corresponding LPS-dependent IL-8 release; LPS-dependent p65 abundance was used as a positive control28 (Figure 3E).
The extended survival of conditioned neutrophils in vitro allows their genetic manipulation. (A) pmaxGFP plasmid (2 μg) nucleofection (Y-01 program) was performed on freshly purified neutrophils conditioned for 20 hours prior to analysis. Cells were imaged by confocal microscopy (whole neutrophil population) detecting GFP (green), Annexin V (Red), and DNA (PI, blue). Bar represents 60 μm. (B) The transfection efficiency was measured by flow cytometry on the Annexin−/PI− population (left) comparing conditioned or unconditioned neutrophils (21% O2, no supplementation) (right). Error bars indicate SD. ***P < .001 (n = 3). (C) The neutrophil viability (Annexin V−/PI−) and absolute cell count were calculated on control (untransfected) and transfected neutrophils (unconditioned/conditioned). Error bars indicate SD. ***P < .001 (n = 3). (D) The efficiency of siRNA transfection on conditioned neutrophils was evaluated on the LPS-stimulated IL-8 expression model. IL-8 mRNA expression was quantified upon stimulation with 10 ng/mL LPS during 3 hours at 37°C on freshly purified and conditioned neutrophils by quantitative reverse transcription polymerase chain reaction. Similar LPS stimulation was performed on neutrophils nucleofected with IL-8 siRNA or negative control siRNA (1 μM). IL-8 mRNA expression fold change was calculated compared with freshly purified neutrophils. Error bars indicate SD. “ns” indicates P > .05; ***P < .001, (n = 3). (E) Enzyme-linked immunosorbent assay quantification of IL-8 release in the supernatant fractions (Sup.) of neutrophils populations described in panel D. As controls, p65 and actin were detected by western blot in cellular (Cell.) fractions. Error bars indicate SD. ***P < .001; “ns” indicates P > .05 (n = 3).
The extended survival of conditioned neutrophils in vitro allows their genetic manipulation. (A) pmaxGFP plasmid (2 μg) nucleofection (Y-01 program) was performed on freshly purified neutrophils conditioned for 20 hours prior to analysis. Cells were imaged by confocal microscopy (whole neutrophil population) detecting GFP (green), Annexin V (Red), and DNA (PI, blue). Bar represents 60 μm. (B) The transfection efficiency was measured by flow cytometry on the Annexin−/PI− population (left) comparing conditioned or unconditioned neutrophils (21% O2, no supplementation) (right). Error bars indicate SD. ***P < .001 (n = 3). (C) The neutrophil viability (Annexin V−/PI−) and absolute cell count were calculated on control (untransfected) and transfected neutrophils (unconditioned/conditioned). Error bars indicate SD. ***P < .001 (n = 3). (D) The efficiency of siRNA transfection on conditioned neutrophils was evaluated on the LPS-stimulated IL-8 expression model. IL-8 mRNA expression was quantified upon stimulation with 10 ng/mL LPS during 3 hours at 37°C on freshly purified and conditioned neutrophils by quantitative reverse transcription polymerase chain reaction. Similar LPS stimulation was performed on neutrophils nucleofected with IL-8 siRNA or negative control siRNA (1 μM). IL-8 mRNA expression fold change was calculated compared with freshly purified neutrophils. Error bars indicate SD. “ns” indicates P > .05; ***P < .001, (n = 3). (E) Enzyme-linked immunosorbent assay quantification of IL-8 release in the supernatant fractions (Sup.) of neutrophils populations described in panel D. As controls, p65 and actin were detected by western blot in cellular (Cell.) fractions. Error bars indicate SD. ***P < .001; “ns” indicates P > .05 (n = 3).
Pro- and antiapoptotic pathways modulate conditioned neutrophil viability
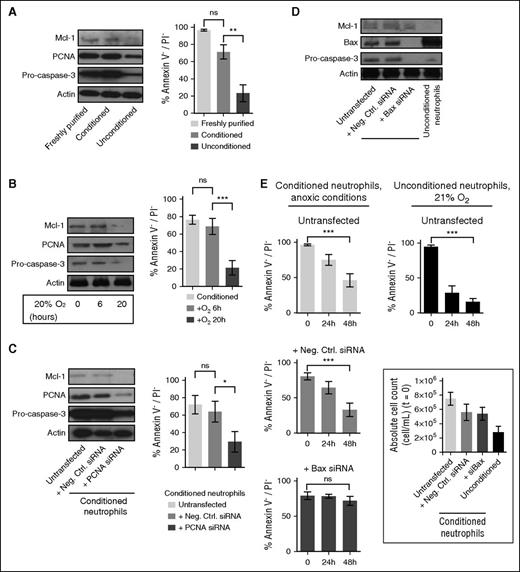
Key antiapoptotic proteins involved in neutrophil viability regulation, such as Mcl-129 and PCNA,30 were stabilized in conditioned neutrophils, together with pro-caspase-3 (Figure 4A), when the reoxygenation of conditioned neutrophils reduced their survival and led to a destabilization of Mcl-1, PCNA, and pro-caspase-3 (Figure 4B). PCNA siRNA nucleofection of conditioned neutrophils was efficient (reduction of PCNA abundance) and increased neutrophil apoptosis 20 hours posttransfection (Figure 4C), confirming its antiapoptotic function.30 Consistently, Bax siRNA nucleofection in conditioned neutrophils reduced Bax abundance (Figure 4D) and decreased neutrophil apoptosis 48 hours posttransfection, further extending conditioned neutrophil viability (Figure 4E).
Conditioned neutrophil viability is reversible upon reoxygenation and can be modulated by targeting PCNA or Bax expression. (A) Mcl-1, PCNA, and pro-caspase-3 stability was assessed by western blot on freshly purified and conditioned/unconditioned neutrophils (20 hours). Neutrophil viability was quantified by flow cytometry. Error bars indicate SD. **P < .01; “ns” indicates P > .05 (n = 5). (B) Conditioned neutrophils were subsequently exposed to 21% O2 for 6 and 20 hours. Mcl-1, PCNA, and pro-caspase-3 were detected by western blot, and neutrophil viability was assessed by flow cytometry. Error bars indicate SD. ***P < .001; “ns” indicates P > .05 (n = 5). (C-E) Conditioned neutrophils were transfected with negative control (Neg. Ctrl.) siRNA, PCNA siRNA (C), or Bax siRNA (D); Mcl-1, PCNA, and pro-caspase-3 stability was analyzed by western blot 20 hours (C) or 48 hours (D-E) postnucleofection (after nucleofection, neutrophils were cultured in RMPI supplemented with 10 mM HEPES and 3 mM glucose for the indicated period of time). Neutrophil viability was quantified by flow cytometry (C, E). Error bars indicate SD. ***P < .001; **P < .01; “ns” indicates P > .05 (n = 5).
Conditioned neutrophil viability is reversible upon reoxygenation and can be modulated by targeting PCNA or Bax expression. (A) Mcl-1, PCNA, and pro-caspase-3 stability was assessed by western blot on freshly purified and conditioned/unconditioned neutrophils (20 hours). Neutrophil viability was quantified by flow cytometry. Error bars indicate SD. **P < .01; “ns” indicates P > .05 (n = 5). (B) Conditioned neutrophils were subsequently exposed to 21% O2 for 6 and 20 hours. Mcl-1, PCNA, and pro-caspase-3 were detected by western blot, and neutrophil viability was assessed by flow cytometry. Error bars indicate SD. ***P < .001; “ns” indicates P > .05 (n = 5). (C-E) Conditioned neutrophils were transfected with negative control (Neg. Ctrl.) siRNA, PCNA siRNA (C), or Bax siRNA (D); Mcl-1, PCNA, and pro-caspase-3 stability was analyzed by western blot 20 hours (C) or 48 hours (D-E) postnucleofection (after nucleofection, neutrophils were cultured in RMPI supplemented with 10 mM HEPES and 3 mM glucose for the indicated period of time). Neutrophil viability was quantified by flow cytometry (C, E). Error bars indicate SD. ***P < .001; **P < .01; “ns” indicates P > .05 (n = 5).
Conditioned neutrophils are efficiently transfused
Neutrophil transfusion efficiency depends on the number and the viability of transfused cells.8,9 We aimed to further confirm that conditioned neutrophils were functional in vivo, in a preclinical study. Guinea pig (Figures 5A-C) or human (Figures 5D,G) conditioned neutrophils were transfused to neutropenic guinea pigs (naïve or infected with S. flexneri). Considering that neutrophil reoxygenation alters the conditioning benefits on their survival maintenance (Figure 4B), neutrophils were manipulated without atmospheric exposure prior to transfusion. Compared with unconditioned neutrophils, bacterial clearance (Figure 5B) and MPO activity (Figure 5C) in infected colonic tissue were increased upon transfusion of guinea pig conditioned neutrophils. Similar results were obtained upon the transfusion of human conditioned neutrophils, with lower activities (Figures 5D-E).
The extended survival of conditioned neutrophils increases the neutrophil transfusion efficiency. (A-E) Conditioned guinea pig or human neutrophil transfusion in a guinea pig model of S. flexneri colonic infection. Neutropenia was produced in guinea pig by cyclophosphamide injections. S. flexneri green-fluorescent protein expressing strain (pGFP) was inoculated intrarectally (109 CFUs) in conventional and neutropenic guinea pigs. When indicated, neutropenic animals were transfused 1 hour postinfection with purified guinea pig (A-C) or human (D-E) neutrophils (freshly purified, conditioned/unconditioned [20 hours]). Animals were euthanized 8 hours postinfection, and tissues were collected. (A) Confocal imaging of conventional or neutropenic guinea pig colonic mucosa infected by S. flexneri pGFP upon guinea pig neutrophil transfusion: S. flexneri pGFP (green), neutrophils (MMP-9, Red), and DNA (Dapi, blue). Bars represent 20 μm. (B-D) Living S. flexneri was counted in tissues by quantifying CFUs per gram of tissue. (C-E) MPO activity in infected tissues (U/mg) was quantified. Error bars indicate SD. ***P < .001; *P < .05; “ns” indicates P > .05 (n = 3). (F-G) Percentage of viable human neutrophils recovered in guinea pig blood circulation 8 hours posttransfusion. Human neutrophils (107) were transfused in neutropenic guinea pig. Eight hours posttransfusion, circulating neutrophils were purified, and viable (PI−) neutrophils were counted by flow cytometry. The % CD15/PI− rates were averaged from 4 animals per condition. (F) Recovery rates were calculated upon freshly purified, conditioned, or unconditioned neutrophil transfusion. (G) Recovery rates were calculated upon conditioned neutrophil untransfected or transfected (siCtrl, siPCNA, siBax) transfusion. Error bars indicate SD. ***P < .001; “ns” indicates P > .05 (n = 4).
The extended survival of conditioned neutrophils increases the neutrophil transfusion efficiency. (A-E) Conditioned guinea pig or human neutrophil transfusion in a guinea pig model of S. flexneri colonic infection. Neutropenia was produced in guinea pig by cyclophosphamide injections. S. flexneri green-fluorescent protein expressing strain (pGFP) was inoculated intrarectally (109 CFUs) in conventional and neutropenic guinea pigs. When indicated, neutropenic animals were transfused 1 hour postinfection with purified guinea pig (A-C) or human (D-E) neutrophils (freshly purified, conditioned/unconditioned [20 hours]). Animals were euthanized 8 hours postinfection, and tissues were collected. (A) Confocal imaging of conventional or neutropenic guinea pig colonic mucosa infected by S. flexneri pGFP upon guinea pig neutrophil transfusion: S. flexneri pGFP (green), neutrophils (MMP-9, Red), and DNA (Dapi, blue). Bars represent 20 μm. (B-D) Living S. flexneri was counted in tissues by quantifying CFUs per gram of tissue. (C-E) MPO activity in infected tissues (U/mg) was quantified. Error bars indicate SD. ***P < .001; *P < .05; “ns” indicates P > .05 (n = 3). (F-G) Percentage of viable human neutrophils recovered in guinea pig blood circulation 8 hours posttransfusion. Human neutrophils (107) were transfused in neutropenic guinea pig. Eight hours posttransfusion, circulating neutrophils were purified, and viable (PI−) neutrophils were counted by flow cytometry. The % CD15/PI− rates were averaged from 4 animals per condition. (F) Recovery rates were calculated upon freshly purified, conditioned, or unconditioned neutrophil transfusion. (G) Recovery rates were calculated upon conditioned neutrophil untransfected or transfected (siCtrl, siPCNA, siBax) transfusion. Error bars indicate SD. ***P < .001; “ns” indicates P > .05 (n = 4).
The throughout recovery of human neutrophils in the guinea pig blood flow was evaluated 8 hours posttransfusion. Upon transfusion of freshly purified or conditioned human neutrophils in naïve guinea pigs, similar viable neutrophil recovery rates were observed (23 ± 11% vs 19 ± 7%) 8 hours posttransfusion (Figure 5F). These throughout recovery rates were higher than upon unconditioned neutrophil transfusion (5 ± 3%) (Figure 5F). The recovery of transfused neutrophils in this model was decreased upon PCNA siRNA nucleofection and increased upon Bax siRNA nucleofection (Figure 5G), confirming in vivo the importance of these 2 proteins in the conditioned neutrophil viability modulation characterized in vitro (Figure 4C-E).
Discussion
Neutrophils have long been termed “short-lived cells.”31 Our results suggest that this assumption is largely dependent on the conditions of their manipulation in vitro rather than on their actual life span in vivo, which remains under discussion.2,3 In this report, we showed that limiting the exposure of neutrophils to atmospheric levels of oxygen increased their viability (Figure 1A-B), consistent with previous reports Walmsley et al.10,13 In anoxic conditions, neutrophil morphological (Figure 1B), physiological, and bactericidal functions (Figures 1C and 2A-D) remained constant. The extended viability of neutrophils under anoxia in the presence of glucose and DMOG allowed efficient transfection with plasmid or siRNA (Figures 3 and 4). In particular, the genetic manipulation of key pro- and antiapoptotic factors (Bax, PCNA) was shown to modulate conditioned neutrophil viability (Figure 4). The reoxygenation of conditioned neutrophils decreased their life span (Figure 4B), justifying the necessity to keep transfused neutrophils away from atmospheric air exposure (Figure 5). This observation is particularly important regarding neutrophil manipulation for transfusion and will have to be considered in the future to improve their therapeutical use. Considering that oxygen has a negative impact on neutrophil viability, further experiments will be required to evaluate the potential beneficial effect of neutrophil collection, purification, and maintenance under anoxic conditions on their viability in vitro and clinical use.
Our results confirm the adaptation of neutrophils in low oxygen conditions and are probably related to the fact that neutrophil energy production mainly relies on glycolysis and not on mitochondrial activity. This assumption is supported by the low abundance of mitochondria in neutrophil cytosol; their activity was found to be restricted to apoptosis induction, not energy production,15,32 but also chemotaxis modulation.33 This physiological characteristic represents a fundamental difference between neutrophils and platelets or lymphocytes.34
In this study, the functional characterization of conditioned neutrophils was achieved under atmospheric conditions, in order to compare the results with standard assays. Further experiments will be required to better understand the role of oxygen on the modulation of neutrophil function, particularly in relation to mitochondria abundance and activity, as previously reported.33
Up to now, studies addressing the adaptation of neutrophils to hypoxia or anoxia were restricted to inflammatory or infectious diseases.13,35,36 However, under physiological conditions, neutrophils naturally evolve in low oxygen environments. Neutrophil maturation from hematopoietic stem cells occurs in the bone marrow, which is a hypoxic environment.37,38 In this context, maturating cells produce energy through glycolysis rather than respiration in an HIF-1–dependent manner.39,40 Once released into the blood circulation through sinusoids, neutrophils will circulate in the blood plasma fraction. The blood circulation is characterized as an oxygenated compartment. However, the blood plasma contains low levels of dissolved oxygen, estimated to be ∼2% of the total oxygen transported by red blood cells.41 Considering that the arterial pO2 equals 75 to 100 mmHg and the venous pO2 equals 30 to 50 mmHg,42 the plasmatic pO2 equals 0.3 to 1 mmHg (0.04% to 0.13% O2), which is quasi-anoxic.
In conclusion, neutrophils are very sensitive to oxygen, the abundance of which is limited in vivo under physiological conditions. This fundamental aspect will have to be further integrated into in vitro models to improve the characterization of neutrophil physiology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Valérie Lapierre and Dominique Tramalloni (Laboratoire de Thérapie Cellulaire, Gustave Roussy Cancer Campus, Villejuif, France) for supplying blood samples from G-CSF–stimulated patients.
This work was supported by grants from the European Union’s European Research Council (HOMEOPITH, 272398) and the European Union’s Seventh Framework Programme (TORNADO, 222720) (B.S.M. and P.S.J.), and by a principal investigator award from Science Foundation Ireland (C.T.T.).
Authorship
Contribution: V.M., M.-C.P., C.C.-L., and B.S.M. performed the research and together with M.-N.U. provided healthy donors’ blood samples; C.C., C.T.T., V.W.-S., and P.J.S. interpreted data; and B.S.M. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benoit S. Marteyn, Institut Pasteur, Unité PMM, 28 rue du Dr Roux, 75724 Paris Cedex 15, France; e-mail: marteyn@pasteur.fr.

![Figure 5. The extended survival of conditioned neutrophils increases the neutrophil transfusion efficiency. (A-E) Conditioned guinea pig or human neutrophil transfusion in a guinea pig model of S. flexneri colonic infection. Neutropenia was produced in guinea pig by cyclophosphamide injections. S. flexneri green-fluorescent protein expressing strain (pGFP) was inoculated intrarectally (109 CFUs) in conventional and neutropenic guinea pigs. When indicated, neutropenic animals were transfused 1 hour postinfection with purified guinea pig (A-C) or human (D-E) neutrophils (freshly purified, conditioned/unconditioned [20 hours]). Animals were euthanized 8 hours postinfection, and tissues were collected. (A) Confocal imaging of conventional or neutropenic guinea pig colonic mucosa infected by S. flexneri pGFP upon guinea pig neutrophil transfusion: S. flexneri pGFP (green), neutrophils (MMP-9, Red), and DNA (Dapi, blue). Bars represent 20 μm. (B-D) Living S. flexneri was counted in tissues by quantifying CFUs per gram of tissue. (C-E) MPO activity in infected tissues (U/mg) was quantified. Error bars indicate SD. ***P < .001; *P < .05; “ns” indicates P > .05 (n = 3). (F-G) Percentage of viable human neutrophils recovered in guinea pig blood circulation 8 hours posttransfusion. Human neutrophils (107) were transfused in neutropenic guinea pig. Eight hours posttransfusion, circulating neutrophils were purified, and viable (PI−) neutrophils were counted by flow cytometry. The % CD15/PI− rates were averaged from 4 animals per condition. (F) Recovery rates were calculated upon freshly purified, conditioned, or unconditioned neutrophil transfusion. (G) Recovery rates were calculated upon conditioned neutrophil untransfected or transfected (siCtrl, siPCNA, siBax) transfusion. Error bars indicate SD. ***P < .001; “ns” indicates P > .05 (n = 4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/7/10.1182_blood-2015-11-680918/6/m_993f5.jpeg?Expires=1765894545&Signature=B1YqVFSOVCb76gm-1To~DLrpv5DUQp06iokJWSPi-NGC9X6xK63ScM7LPGwGKJrC9Cpo0fjTUxCXwmcVnHWQ9vu93qPzYg-f1jDb5qKMfyghxyHDG4gCB~aP0XuY9uhZh992bdd65B81m2-LcjQ0fjMV54fXAdQ5V5kC2E2xCeMuOhk0OhT0Ru~ox2xA7dbfyWb1JEgv0TUbkVWiHezUZ2TrF~sdoNkRRbdF3NePvKRRFToI44S8y~jPoxPRqJT0rrftu02MswlMPBR65v49AAttHm5nWg27uZTCOSdSzUVdpn5ifzuG5n4lJWq7D9tOKC79vZD-bhPfvaCzOBjtnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal