To the editor:
Familial hemophagocytic lymphohistiocytosis type 3 (FHL3) is a hyperinflammatory disease caused by mutations in the UNC13D gene (coding for the Munc13-4 protein); FHL3 accounts for 30% to 35% of FHL cases.1 In cytotoxic T cells (CTLs) and natural killer cells, Munc13-4 helps to prime perforin-containing, cytotoxic granules before they fuse with the plasma membrane at the immunological synapse.2,3 In the absence of effective cytotoxicity, antigen-presenting cells continue to stimulate CTLs, leading to massive proliferation of the latter, the excessive production of interferon γ (IFNγ), macrophage hyperactivation, and tissue infiltration by activated cells. The clinical phenotype is characterized by prolonged fever, hepatosplenomegaly, lymphadenopathy, rashes, and edema.4 The condition often appears to be triggered by a viral infection (Epstein-Barr virus [EBV], in particular).5-7
Hematopoietic stem cell transplantation (HSCT) is the only available curative option for FHL3; however, the posttreatment overall survival rate is not satisfactory and depends on (1) the disease state prior to HSCT and (2) the availability of a matched sibling donor.4 In addition to HSCT, gene therapy of hematopoietic stem cells has been tested as a treatment of FHL2 in a murine model.8,9 Given that the main defect in FHL disease is cytotoxic dysfunction of mature T cells, the latter constitute a potentially valuable target for gene therapy approaches. The genetic modification of T cells has produced remarkable clinical outcomes in cancer immunotherapy and in cases of adenosine deaminase deficiency.10,11 To investigate the feasibility and efficacy of gene transfer into human FHL3 T cells as a means of gene therapy for FHL3, we transduced prestimulated patients’ T cells with a measles virus H and F glycoprotein-pseudotyped lentiviral vector (H/F-LV) prior to adoptive transfer in a NSG mouse model bearing EBV-induced lymphoma. The cytotoxic activity of gene-corrected T cells was restored, as evidenced by an increase in their in vitro degranulation capacity and a progressive regression in the mass of EBV lymphoma (due to the efficient homing of functional cytotoxic T cells in vivo). Transduction of T cells from patients with FHL3 with a conventional vesicular stomatitis virus-G lentiviral vector (VSVG-LV) also restored the degranulation capacity (albeit with a lower transduction efficiency than H/F-LV). T memory stem cells (TSCM) were also successfully transduced, and maintained their stem characteristics throughout the culture period. The present work is the first to highlight the potential of engineered T-cell gene therapy in a context of FHL.
Activated peripheral blood mononuclear cells (PBMCs) from FHL3 patients were transduced with either H/F-LV at multiplicity of infection (MOI) of 5 or with VSVG-LV at MOIs of 5 and 100. Both vectors code for a human Munc13-4/cyan fluorescent protein (CFP) fusion protein (supplemental Figure 1A, available on the Blood Web site).
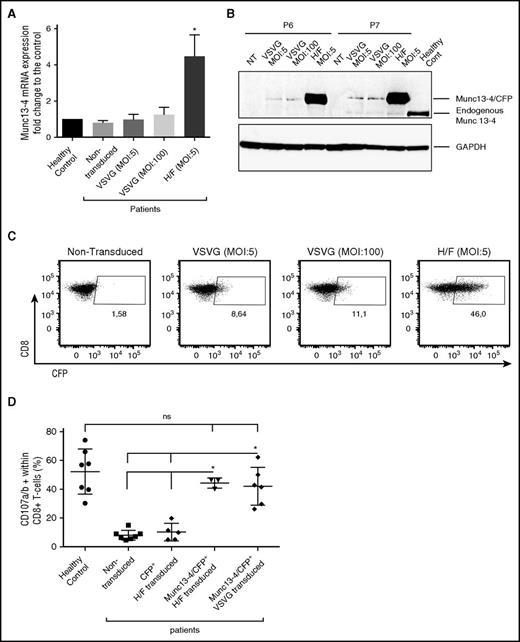
An evaluation of the transduction efficacy in bulk transduced cells (6 days after transduction) revealed that the mean vector copy number was higher for H/F-LV than for VSVG-LV (2.22 for H/F-LV; 0.15 and 0.35 for VSVG-LV at MOIs of 5 and 100, respectively) and that Munc13-4 messenger RNA (mRNA) (Figure 1A) and protein (Figure 1B) expression levels were higher in bulk-H/F-LV–transduced cells. The CFP expression on CD8+ gated cells reached a value of 47.8% ± 13% for H/F-LV but only 7.06% ± 2.4% for VSVG-LV at an MOI of 5 and 12.5% ± 2.5% at an MOI of 100 (Figure 1C).
In vitro restoration of Munc13-4 expression and cytotoxic function after gene transfer into Munc13-4–deficient T cells. (A) The level of Munc13-4 mRNA expression was 4.4 to 5.5 times higher in the patients’ H/F-LV-bulk–transduced cells than in healthy controls and nontransduced FHL3 cells, and was 1.5 times higher in VSVG-LV–transduced cells (MOI, 100) than in nontransduced cells. The experiments were performed on 3 different FHL3 samples (P5, P6, and P7). The data were normalized against GAPDH and are expressed as a fold-change relative to healthy control T cells. It is noteworthy that mRNA levels were similar in nontransduced patient cells and healthy control cells. (B) Munc13-4 protein expression 6 days after transduction (in the same 3 samples as described for panel A; only P6 and P7 are presented here). (C) Dot plots represent (for CD8+ gated cells) the number of Munc13-4/CFP+ cells as a percentage of the CD8+ population 6 days after transduction with H/F-LV at an MOI of 5 or with VSVG-LV at MOIs of 5 and 100; 1 representative experiment of 3 (same samples as in panel A) is shown. (D) The number of CD107a/b+ cells as a percentage of the CD8+ T-cell population after stimulation with 30 µg/mL anti-CD3. In transduced samples, the percentage of CD107a/b+ cells are presented with respect to CFP+ or Munc13-4/CFP+ gated cells. n = 7 different FHL3 samples for nontransduced cells (P1-P7); n = 5 for H/F-CFP as control (P3-P7); n = 3 for H/F (P5-P7); n = 6 for VSVG experiments (P1-P3, P5-P7). Data are presented as the mean ± standard deviation (SD). P values were calculated using an unpaired Student t test for mRNA expression and a 2-sided Mann-Whitney test for CD107a/b surface expression in the degranulation assay. *P < .05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, nonsignificant; NT, nontransduced.
In vitro restoration of Munc13-4 expression and cytotoxic function after gene transfer into Munc13-4–deficient T cells. (A) The level of Munc13-4 mRNA expression was 4.4 to 5.5 times higher in the patients’ H/F-LV-bulk–transduced cells than in healthy controls and nontransduced FHL3 cells, and was 1.5 times higher in VSVG-LV–transduced cells (MOI, 100) than in nontransduced cells. The experiments were performed on 3 different FHL3 samples (P5, P6, and P7). The data were normalized against GAPDH and are expressed as a fold-change relative to healthy control T cells. It is noteworthy that mRNA levels were similar in nontransduced patient cells and healthy control cells. (B) Munc13-4 protein expression 6 days after transduction (in the same 3 samples as described for panel A; only P6 and P7 are presented here). (C) Dot plots represent (for CD8+ gated cells) the number of Munc13-4/CFP+ cells as a percentage of the CD8+ population 6 days after transduction with H/F-LV at an MOI of 5 or with VSVG-LV at MOIs of 5 and 100; 1 representative experiment of 3 (same samples as in panel A) is shown. (D) The number of CD107a/b+ cells as a percentage of the CD8+ T-cell population after stimulation with 30 µg/mL anti-CD3. In transduced samples, the percentage of CD107a/b+ cells are presented with respect to CFP+ or Munc13-4/CFP+ gated cells. n = 7 different FHL3 samples for nontransduced cells (P1-P7); n = 5 for H/F-CFP as control (P3-P7); n = 3 for H/F (P5-P7); n = 6 for VSVG experiments (P1-P3, P5-P7). Data are presented as the mean ± standard deviation (SD). P values were calculated using an unpaired Student t test for mRNA expression and a 2-sided Mann-Whitney test for CD107a/b surface expression in the degranulation assay. *P < .05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, nonsignificant; NT, nontransduced.
The surface expression of CD107a/b after T-cell receptor (TCR) stimulation (which accompanies the release of cytotoxic granules) indicated that transduced CD8+ T cells expressing Munc13-4/CFP (Munc13-4/CFP+) had recovered normal (healthy control) levels of granule release capacity (Figure 1D). This result also suggested that the fusion of CFP with Munc13-4 did not alter the protein’s functionality.
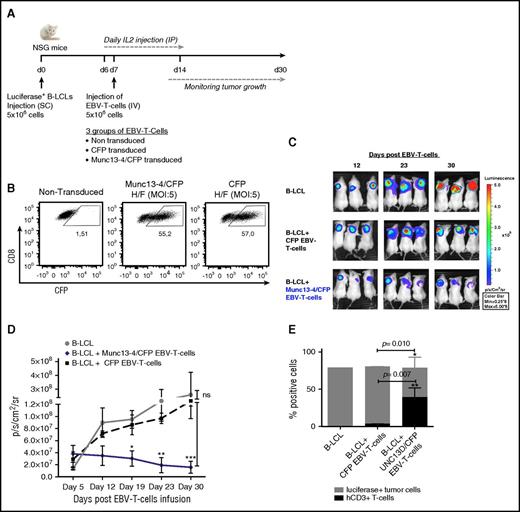
On the basis of these in vitro data, we measured the in vivo cytotoxic activity of Munc13-4/CFP–transduced T cells from FHL3 patients. EBV is one the major infectious triggers of hemophagocytic lymphohistiocytosis (HLH). We therefore decided to test the ability of Munc13-4/CFP–transduced T cells to eliminate EBV-induced B-cell lymphoma in vivo. Briefly, NSG mice were transplanted with a luciferase-expressing, EBV-transformed B- lymphoblastoid cell line (B-LCL) derived from a FHL3 patient (P5). When tumors were palpable (around 7 days later), NSG mice were transplanted with EBV-specific T cells from a single FHL3 patient (bulk-transduced with either H/F-LV–expressing Munc13-4/CFP or H/F-LV–expressing CFP) (Figure 2A). Measurements of CFP expression 7 days after transduction (ie, before the infusion of EBV-T cells into the mice) showed that up to 55.2% of the cells had been transduced (Figure 2B) and that the Munc13-4 mRNA level was 5 times higher in bulk-transduced cells than in healthy controls (data not shown). As shown in Figure 2C-D, and as observed previously12 using the same xenograft model but with a healthy control as a donor, the tumor mass declined progressively and significantly in NSG mice transplanted with Munc13-4/CFP–transduced T cells. This contrasted with the outcomes in nontransplanted mice and in mice transplanted with CFP-transduced T cells, in which the tumors continued to grow up to 30 days posttransplantation. Immunostaining and flow cytometry analysis revealed massive lymphocytic tumor infiltration in NSG mice transplanted with Munc13-4/CFP–transduced T cells (Figure 2E; supplemental Figure 1B). These results clearly demonstrate that transduction with a LV containing the UNC13D gene restores not only the expression of Munc13-4 protein but also the in vitro granule release capacity and in vivo cytotoxic function of T cells from FHL3 patients.
Adoptive transfer of Munc13-4–corrected EBV-specific T cells induces the regression of EBV B-cell lymphoma in NSG mice. (A) Experimental design for the xenograft NSG mouse model. The experiment was performed with PBMCs from P5 (an EBV-seropositive patient). N = 3 mice per group. (B) The dot plot represents the number of Munc13-4/CFP+ or CFP+ cells as a percentage of the CD8+-gated population 7 days after transduction. (C) Bioimmunoluminescence imaging using the IVIS in vivo imaging system (Xenogen; Caliper Life Sciences, Hopkinton, MA) for NSG mice bearing EBV B-cell lymphoma. EBV-T cells, EBV-specific cytotoxic T cells. (D) The time course of tumor growth. Photon emission from luciferase-positive tumor cells was quantified as the peak photons per second per cm2 per steradian (p/s/cm2/sr). Error bars represent the mean ± standard error of the mean (SEM). The P value was calculated in a 2-way analysis of variance with a Bonferroni posttest. (E) The percentage of human CD3+ (hCD3+) T cells and luciferase-expressing lymphoma cells in digested tumors, as measured by flow cytometry. The mean cell count ± SD is shown. The P value was calculated in an unpaired Student t test. *P < .05; **P < .01; ***P < .001. IP, intraperitoneal; SC, subcutaneous.
Adoptive transfer of Munc13-4–corrected EBV-specific T cells induces the regression of EBV B-cell lymphoma in NSG mice. (A) Experimental design for the xenograft NSG mouse model. The experiment was performed with PBMCs from P5 (an EBV-seropositive patient). N = 3 mice per group. (B) The dot plot represents the number of Munc13-4/CFP+ or CFP+ cells as a percentage of the CD8+-gated population 7 days after transduction. (C) Bioimmunoluminescence imaging using the IVIS in vivo imaging system (Xenogen; Caliper Life Sciences, Hopkinton, MA) for NSG mice bearing EBV B-cell lymphoma. EBV-T cells, EBV-specific cytotoxic T cells. (D) The time course of tumor growth. Photon emission from luciferase-positive tumor cells was quantified as the peak photons per second per cm2 per steradian (p/s/cm2/sr). Error bars represent the mean ± standard error of the mean (SEM). The P value was calculated in a 2-way analysis of variance with a Bonferroni posttest. (E) The percentage of human CD3+ (hCD3+) T cells and luciferase-expressing lymphoma cells in digested tumors, as measured by flow cytometry. The mean cell count ± SD is shown. The P value was calculated in an unpaired Student t test. *P < .05; **P < .01; ***P < .001. IP, intraperitoneal; SC, subcutaneous.
In a clinical trial of T-cell gene therapy for adenosine deaminase deficiency, the transduced T cells persisted in the circulation for up to 12 years after infusion13 as a result of efficient transduction, in vitro expansion, and the in vivo persistence of TSCM. In the present study, we confirmed the presence of TSCM within the T-cell pool from FHL3 patients (supplemental Figure 2A). We also demonstrated that TSCM were transduced by both H/F- and VSVG-LVs (supplemental Figure 2B). The proportion of TSCM increased 48 hours after TCR stimulation and decreased between days 2 and 8 of culture as a result of differentiation into other memory cells. However, a fraction of these TSCM was still detectable at day 8 in both nontransduced and transduced conditions, whereas no naive cells were found in the culture (supplemental Figure 2C).
Our study is the first to highlight the potential of T-cell gene therapy in a context of FHL. Targeting mature T cells (rather than hematopoietic stem cells) is associated with a lower risk of insertional mutagenesis and cell transformation.14 However, one of the limitations of this strategy relates to the generally low transductional efficacy of VSVG-LV. The post-HSCT outcome in HLH patients shows that remission is maintained as long as the level of T-cell donor chimerism reaches 10% to 15%.15,16 The conventional VSVG-LV strategy must therefore be tested in a preclinical murine model, in order to establish whether or not poor in vitro T-cell transduction prevents the achievement of this level of chimerism in vivo. Another limitation relates to the availability of T cells as a result of pancytopenia and the manifestations of HLH. Novel anti-inflammatory modalities for IFNγ blockade (such as the JAK1/2 inhibitor ruxolitinib) can reduce inflammation and correct certain manifestations of FHL (such as blood cytopenia) in murine models of FHL.17,18 Ruxolitinib has already demonstrated clinical efficacy in other inflammatory conditions19,20 ; if approved in HLH patients, this compound could be used as an anti-inflammatory agent for reducing immune system imbalance prior to T-cell immunotherapy.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank the patients and their relatives for providing samples. The authors also thank the staff at the Immunology and Paediatric Haematology Department at Necker Children’s Hospital (Paris, France) and Dr G. DeAngulo from Nicklaus Children’s Hospital (Miami, FL) who provided patient care.
This work was supported by INSERM, the Association Française contre les Myopathies (AFM; grant 17045), the European Research Council (ERC; Regenerative Therapy, grant 269037), and a European Union FP7 grant (CELL-PID, 261387).
Contribution: T.S. designed and conducted experiments, analyzed data, and wrote the manuscript; J.R. and I.R. designed, performed, and analyzed experiments and read the manuscript; A.D. and A.-C.D. performed and analyzed experiments; E.V. and F.-L.C. provided vectors and read the manuscript; C.L.-P. discussed data and reviewed the manuscript for critical content; G.d.S.B. provided certain patient samples and reviewed the manuscript for critical content; P.A. discussed data; I.A.-S. designed and supervised the overall research and wrote the manuscript; and M.C. designed and supervised the overall research and reviewed the manuscript for critical content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Isabelle André-Schmutz, Imagine Institute, INSERM U1163, 24, Boulevard de Montparnasse, F-75015 Paris, France; e-mail: andre.schmutz@gmail.com.
References
Author notes
J.R. and I.R. contributed equally to this study.