Abstract
Allogeneic hematopoietic stem cell transplantation (Allo-SCT) with fludarabine based reduced intensity or toxicity reduced conditioning regimen with Busulfan or Melphalan (Flu/Bu; Flu/Mel) has improved HSCT outcome in patients with AML. Limited reports on the PK of Flu in patients with malignant hematological diseases undergoing Allo-SCT suggest that Flu AUC is associated with transplant related mortality (TRM) (Long-Boyle et al, 2011). We prospectively analyzed the PK and pharmacogenetics (PG) of Flu and Bu on Allo-SCT outcome in patients with AML receiving Flu/Bu or Flu/Mel based regimen.
Forty-eight adult AML patients receiving Flu/Bu(targeted) (Flu 40mg/m2 /day intravenously from day-5 to -2 and intravenous Bu 130mg/m2 /day from day-5 to-2; n=27) or Flu/Mel (Flu 30mg/m2 /day intravenously from day -6 to -2 and Mel 140mg/m2 intravenously on day -1; n=21) regimen prior to Allo-SCT between January 2012 and 2015 were enrolled in this study. Peripheral blood was collected for PK analysis at serial time points and plasma was separated and analyzed the same day (for Bu TDM). Bu dose was adjusted based on the first dose PK to achieve target cumulative total AUC (22000mmoles*min). Flu plasma levels was analyzed using LC-MS/MS and the concentration was expressed as mmol/ml. Genetic polymorphisms that influence Flu and Bu PK (NT5E 5'-UTR variant rs2295890 and GSTA1*B polymorphism) were screened using pre- HSCT genomic DNA. The influence of Flu/Bu PK and PG on clinical outcome endpoints such as overall survival (OS) and event-free survival (EFS) was evaluated using cox regression analysis.
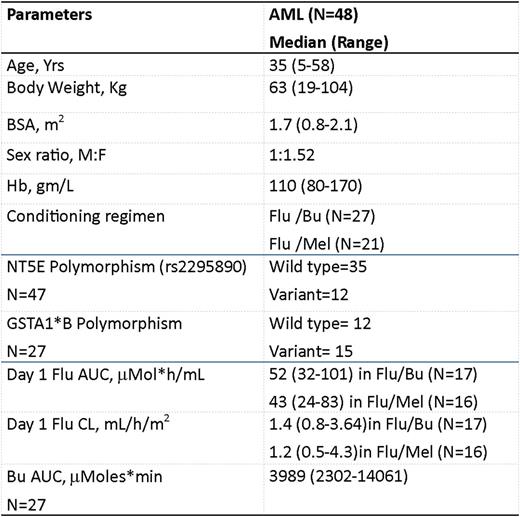
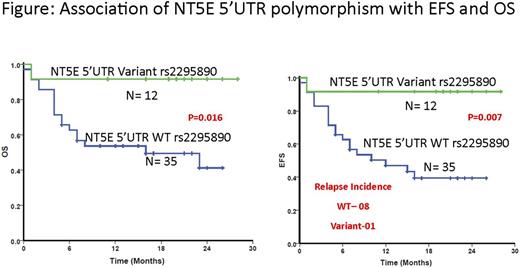
The demographics of the patients is listed in the Table. There was a significant inter-individual variation in the flu clearance (1.2mL/h/m2(0.5-4.3); 8.6 fold) and AUC (46mmol*h/ml (24-101); 4.2 fold). Fludarabine AUC trended higher in Flu/Bu group when compared to Flu/Mel group (median AUC 52 vs. 43 mmol*h/ml respectively; p=0.08). Neither of the polymorphisms significantly explained the variation in Bu or Flu PK in this cohort and none of the PK parameters was associated with EFS/OS. However, patients carrying variant genotype of the NT5E 5'UTR polymorphism (rs2295890) showed significantly better OS (p=0.016) and EFS (p=0.007) (Figure) compared to those carrying wild type genotype. We have previously reported that this variant is associated with decreased Flu Clearance in patients with aplastic anemia and thalassemia receiving Flu based conditioning regimen (Mohanan et al, December 06, 2014; 124 (21): 3884; Mohanan et al, December 03, 2015; 126 (23): 3120). Although not significant, the Flu clearance was lower in the NT5E variants in this cohort as well. It can be extrapolated that patients carrying the variant genotype of the NT5E polymorphism, have decreased relapse incidence (Wild type-8; Variants-1) and hence better EFS and OS post HSCT, due to decreased clearance and increased systemic exposure to Flu. This finding need to be validated in a larger number and in patients with other disease conditions undergoing HSCT with Flu based regimen, in order to achieve the goal of personalizing conditioning regimen.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal