Abstract
Background: Pev (TAK-924/MLN4924), a novel investigational NAE inhibitor, enhances the anti-leukemic effects of aza in AML cell lines and murine xenografts (Smith et al, Blood 2011). Single-agent pev activity was confirmed in relapsed/refractory AML pts (Swords et al, Br J Haematol 2015). This open-label, multi-center, dose-escalation study (NCT01814826) investigated pev + aza in treatment-naïve older AML pts. Dose-limiting toxicities (DLTs)includedG2 hyperbilirubinemia and G4 AST elevation (n=1 each) at pev 30 mg/m2. The maximum tolerated dose (MTD) for the combination was pev 20 mg/m2 + aza 75 mg/m2 (Swords et al, ASH 2014).We present updated safety/efficacy results for the MTD cohort (fully enrolled).
Methods: Primary objectives included safety and tolerability assessments of pev + aza in addition to defining the MTD. Secondary objectives included pharmacokinetics (PK) and disease response assessments. Treatment-naïve pts ≥60 yrs unlikely to benefit from standard induction therapy (defined by ≥1 of: antecedent hematologic disease; known adverse cytogenetic risk; ECOG PS 2; ≥75 yrs), received pev 20 or 30 mg/m2 IV on d 1, 3 and 5, + fixed-dose aza (75 mg/m2 IV/SC) on d 1-5, 8 and 9, every 28 d until disease progression or unacceptable toxicity. Adverse events (AEs) were assessed per NCI-CTCAE v4.03; response per IWG criteria for AML. Bone marrow samples were collected at screening to assess cytogenetic risk (CALGB) and mutation profile; serial samples for PK analysis were drawn in cycle 1.
Results:
Demographics:As of May 17 2016, 61 pts (median age 75 yrs [range 61-89]; 54% male; 77% ECOG PS 0/1, 23% ECOG PS 2; 57% de novo, 43% secondary AML; median marrow blasts 36% [range 5-92]) had received pev 20 mg/m2, of whom 48% had intermediate-, 30% adverse-, and 3% favorable-risk cytogenetics.
Safety/PK: Pts received a median of 4 cycles (range 1-33), and 23/61 pts (38%) received ≥6 cycles of pev + aza. The most common AEs were constipation (46%), nausea (44%), fatigue (43%), and anemia (39%). Fifty pts (82%) experienced ≥G3 AEs; the most frequent (≥15%) were anemia, febrile neutropenia (each 28%), thrombocytopenia (21%), neutropenia (18%), and pneumonia (15%). ≥G3 AST/ALT elevations were reported in 5% of pts. Forty-one pts (67%) experienced serious AEs; the most frequent (≥10%) were febrile neutropenia, neutropenia (each 25%), and pneumonia (11%). Two pts discontinued due to pev related toxicity (G3 febrile neutropenia). There were 11 on-study deaths unrelated to study therapy. In the MTD expansion phase (n=55), 2 pts experienced DLTs of transient G3/4 transaminase elevations, and were successfully re-challenged following dose reduction to remain on study. Pev PK was not altered by the addition of aza.
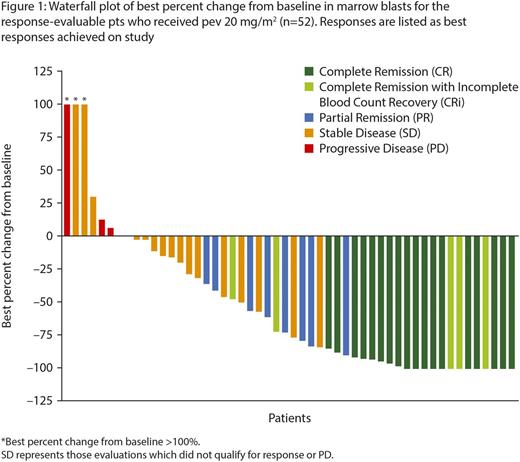
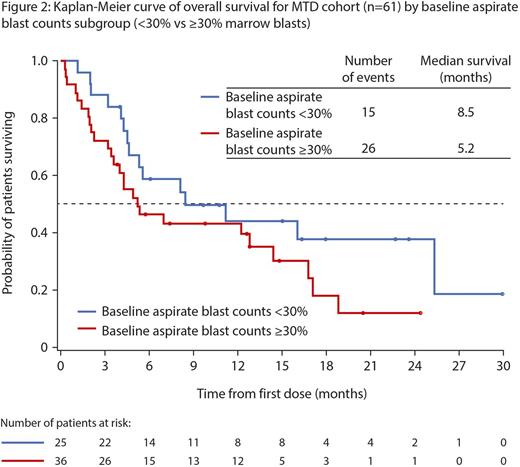
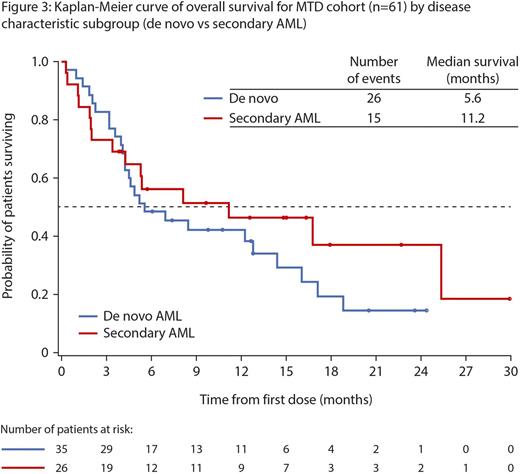
Responses: Overall response rate (ORR) in 52 response-evaluable pts was 60% (18CR, 5CRi, 8PR; Figure 1), with a median duration of remission of 8.3 mos (95% CI: 5.75, 12.06); 19/31 (61%) responses occurred within the first 2 cycles.Of the 23 pts with CR/CRi, 14 had responses lasting ≥4 cycles, 2 went on to have allogeneic stem cell transplant, 9 had intermediate-, 7 adverse-, and 1 favorable-risk cytogenetics. ORR was: 64% (14/22; 7CR, 3CRi, 4PR) vs 57% (17/30; 11CR, 2CRi, 4PR) for pts with low- (<30%) vs high- (≥30%) marrow blasts; 58% (18/31; 11CR, 3CRi, 4PR) vs 62% (13/21; 7CR, 2CRi, 4PR) for de novo vs secondary AML pts; 61% (14/23; 8CR, 1CRi, 5PR) vs 50% (8/16; 5CR, 2CRi, 1PR) for intermediate- vs adverse-risk cytogenetic pts; 83% (19/23; 14CR, 2CRi, 3PR) vs 41% (12/29; 5CR, 3CRi, 4PR) for pts who received ≥6 cycles vs <6 cycles of aza, respectively. Responses were seen in pts with typically refractory disease; 7/11 pts with TP53 mutations achieved either a CR/CRi (n=3) or PR (n=4); 4 stayed on study for >10 cycles. After a median follow-up of 16.4 mos, 6-mo survival was 52%. Median overall survival was: 7.0 mos for the MTD cohort; 8.5 vs 5.2 mos for pts with low- (<30%) vs high- (≥30%) marrow blasts (Figure 2); 5.6 vs 11.2 mos for de novo vs secondary AML pts (Figure 3); and 16.1 vs 5.3 mos for pts aged 65-74 vs ≥75 yrs, respectively.
Conclusion: Pev + aza was well tolerated. Response rates and durable remissions were observed with limited additional toxicity beyond what is expected for aza alone. The timing and frequency of responses suggests benefit from the addition of pev compared to aza alone (Dombret et al, Blood 2015). At the time of writing, a randomized phase 2 study in low-blast AML/high-risk myelodysplastic syndromes is ongoing.
Coutre:Janssen: Consultancy; Pharmacylics, LLC, an AbbVie Company: Consultancy, Research Funding; AbbVie: Research Funding. Zeidner:Tolero: Research Funding; Merck: Research Funding; Takeda: Research Funding; Otsuka: Consultancy; Agios: Honoraria. Foran:medscape: Honoraria; Millennium Pharmaceuticals, Inc.: Research Funding; novartis: Honoraria; pfizer: Honoraria; karyopharm: Honoraria; boehringer ingelheim: Research Funding; agios: Research Funding; Cellerant: Research Funding. Cruz:Millennium Pharmaceuticals, Inc.: Honoraria; Millennium Pharmaceuticals, Inc.: Speakers Bureau. Erba:Novartis: Consultancy, Speakers Bureau; Celator: Research Funding; Daiichi Sankyo: Consultancy; Celgene: Consultancy, Speakers Bureau; Juno: Research Funding; Millennium Pharmaceuticals, Inc.: Research Funding; Pfizer: Consultancy; Seattle Genetics: Consultancy, Research Funding; Incyte: Consultancy, DSMB, Speakers Bureau; Amgen: Consultancy, Research Funding; Agios: Research Funding; Sunesis: Consultancy; Gylcomimetics: Other: DSMB; Astellas: Research Funding; Ariad: Consultancy; Jannsen: Consultancy, Research Funding. Berdeja:Abbvie, Acetylon, Amgen, Bluebird, BMS, Calithera, Celgene, Constellation, Curis, Epizyme, Janssen, Karyopharm, Kesios, Novartis, Onyx, Takeda, Tragara: Research Funding. Tam:Millennium Pharmaceuticals, Inc.: Consultancy. Vardhanabhuti:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Dobler:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Faessel:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Dash:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Sedarati:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Dezube:Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership. Savona:Takeda: Research Funding; Amgen Inc.: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Research Funding; Sunesis: Research Funding; Ariad: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal