Abstract
Background: Allogeneic stem cell transplant (SCT) recipients suffer from a defective T cell mediated immunity causing potentially fatal reactivation of latent viruses. After T cell depleted SCT we have observed over 80% CMV reactivation and significant additional costs ($58-74k/patient). Despite aggressive monitoring and pre-emptive therapy, reactivation and/or positive CMV serology brings a significantly higher risk for non-relapse mortality (NRM). Adoptive transfer of ex vivo generated virus specific donor T cells is effective as a treatment of infection post-SCT but it has not been tested as a prophylaxis of early reactivation. Here in a Phase I study we transferred multi-virus specific T cells (MVSTs) immediately post SCT, targeting CMV, Ebstein-Barr virus (EBV), BK and adenovirus (Ad) as a novel strategy to prevent viral reactivation in the recipients of T cell depleted sibling HLA-matched SCT.
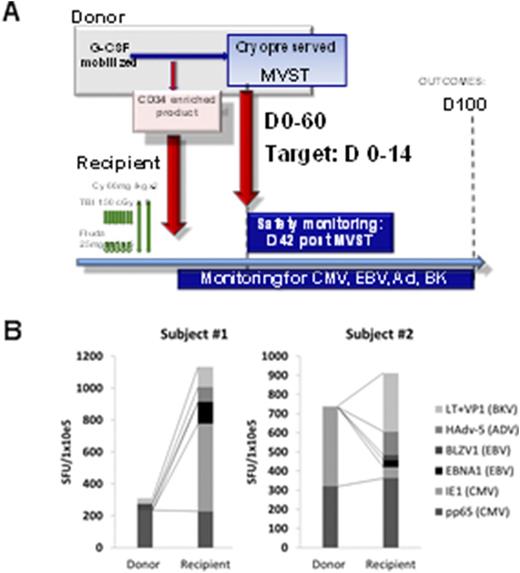
Methods: Subjects were eligible if enrolled in HLA-matched T cell depleted transplant protocol (13-H-0144) and deemed at risk for CMV reactivation. MVST cells were manufactured from SCT sibling donors. Elutriated lymphocytes were stimulated with autologous dendritic cells (DCs) pulsed with seven overlapping peptide libraries (pepmixes) spanning the length of immunodominant proteins from CMV (pp65 and IE1), EBV (BZLF1 and EBNA1), BK (LT and VP1) and Ad5. Cultures were maintained in G-Rex flasks for 14 days in presence of IL-7, IL-15 and IL-2 (after 72hrs), tested for sterility, phenotype, potency and cryopreserved. MVST cells were thawed and administered intravenously as early as possible (day 0 to +60) post SCT. A Phase I 3+3 dose escalation design was used at the following dose levels: Cohort 1 - 1x10e5 total nucleated cells (TNC)/kg, Cohort 2 - 5x10e5 TNC/kg, Cohort 3 - 1x10e6 TNC/kg. The primary safety endpoint at day 42 post infusion was the occurrence of dose limiting toxicity (DLT), (Grade IV GVHD or any other severe adverse even (SAE) deemed to be at least "probably" or "definitely" related to the MVST infusion. Patients were followed to day +100 post SCT for secondary outcomes, including efficacy (Figure) and immune reactivity (for donor/recipient pairs).
Results: MVST cells recognized the majority of pepmixes, were polyfunctional and robustly proliferated in response the cognate antigens, but minimally against allogeneic targets- suggesting a limited ability to induce GVHD. CDR3 sequencing of T cell repertoire showed a significant reduction in diversity and a striking dominance of a limited number of clonotypes in the final MVSTs. Nine subjects were enrolled and treated with MVST cells. MVSTs were successfully generated for all subjects, meeting the release criteria. Median time from SCT to MVST administration was 16 days (range D +6 to +52 post-SCT). Two subjects received MVST after day +30 due to cardiac instability and scheduling. There were no immediate infusion-related adverse events or DLT by day 42. One subject in cohort II developed a self-limiting grade I cytokine release syndrome in the setting of low-level EBV reactivation. One patient (cohort 1) developed de novo grade III aGVHD post-MVST infusion. CMV reactivation post-MVST occurred in 4 out of 8 evaluable subjects (50%) who completed D+100 post-SCT vs. 45 out of 52 patients (50% vs 87%; p value=0.031) in a historical cohort of recipients of T cell depleted SCT. In all cases CMV reactivation occurred during treatment with high dose steroids. In two cases MVST were generated from CMV seronegative donors and showed minimal activity against pp65 and IE1. In eight evaluable subjects who reached D+100 post-SCT there was no EBV-related disease, but we saw self-limiting low level EBV replication in 6 out of 8 cases. There were no cases of BK or Ad-related disease or viremia. ELISPOT analysis at D+100 revealed robust reconstitution of anti-viral immunity in analyzed recipients (vs. donors, Figure, B)
Conclusions: This is the first report demonstrating that it is safe and feasible to use adoptively transferred allo-MVST immediately post-SCT to rapidly reconstitute anti-viral immunity and ameliorate the detrimental impact of the early viral reactivation in SCT recipient. No DLTs were seen. MVSTs had a markedly reduced allo-reactivity and carried a minimal risk of GVHD. Our results also suggest efficacy of this strategy in reducing viral reactivation. A Phase II portion of this study is currently enrolling patients.
Sabatino:Kite: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal