Abstract
Although clonal hematopoiesis of indeterminate potential (CHIP) has been identified as a risk factor for the development of therapy-related myeloid neoplasms (t-MNs), the cellular and molecular mechanisms underlying the adverse effects of chemotherapy on the hematopoietic stem cell (HSC) compartment in patients with CHIP are currently unknown. Understanding these processes is a critical step to clarifying the interaction between CHIP, chemotherapy, and t-MN pathogenesis and identifying earlier therapeutic intervention opportunities in patients who are at high risk of developing these disorders.
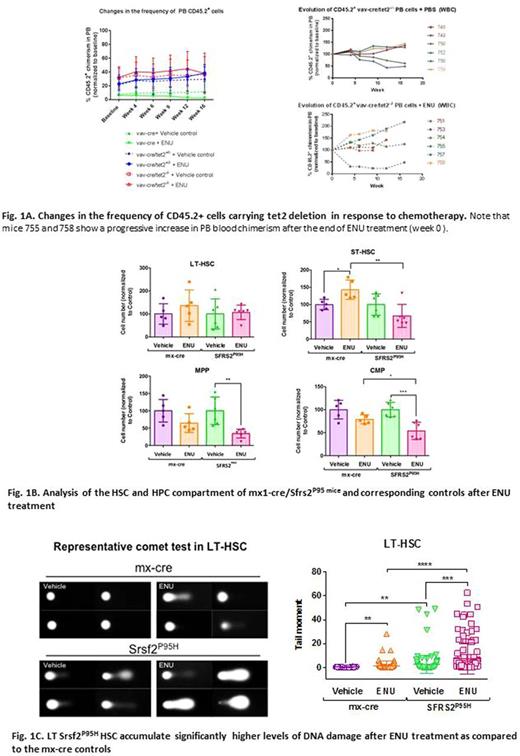
In an attempt to achieve this goal, we first used a bone marrow (BM)-competitive transplantation approach to develop mouse models of CHIP and understand the cellular dynamics of normal and mutant HSC compartments in response to chemotherapy exposure. Specifically, we transplanted low allelic frequencies of CD45.2+ BM cells isolated from mouse models carrying genetic alterations commonly involved in CHIP (such as deletion of tet2 and dnmt3a or the P95 mutation of srsf2) combined with wild-type CD45.1+ BM competitor cells in lethally irradiated wild-type mice. Mice carrying the genetic alterations and corresponding controls received 2 intraperitoneal doses of either N-nitroso-N-ethylurea (ENU; 100 mg/kg), an alkylating agent, or vehicle 9 days apart. Mice showing increased frequency of CD45.2 cells in the peripheral blood (PB) during a window of 12 months after ENU administration were euthanized, and the CD45.2+ BM cells were isolated and analyzed by exome sequencing in comparison to corresponding controls to identify potential genetic aberrations acquired during clonal evolution. Analysis of the BM HSC compartment of mice with increased PB chimerism showed a preferential increase in the frequency of the CD45.2 long-term (LT)-HSC, suggesting that ENU resulted in a competitive advantage for this population. As an example, the clonal evolution relative to each mouse in the untreated and treated vav-cre/tet2-/- groups is shown in Figure 1A.
To understand the molecular mechanisms underlying the competitive advantage of mutant HSCs induced by chemotherapy exposure, we have evaluated whether cells carrying CHIP-related mutations were characterized by an intrinsic difference in chemotherapy susceptibility, which may lead to their clonal evolution. To this end, we treated mx-cre/srsf2P95H and corresponding mx-cre control mice with 2 doses of ENU and analyzed the cellular architecture of the HSC compartment 1 month after treatment (n=5 mice/group). Although the more differentiated mx-cre/srsf2P95H short term (ST)-HSC, multipotent progenitors (MPP), and common myeloid progenitor (CMP) populations showed increased sensitivity to ENU treatment compared to the mx-cre controls, the more primitive mx-cre/srsf2P95H LT-HSC population survived chemotherapy and accumulated significantly higher levels of DNA damage, as measured by the comet assay (Tail moment means [IC95%]: mx1-cre + vehicle 0.32 [0.25-0.39]; mx1-cre + ENU 1.51 [0.74-2.29]; srsf2P95H + vehicle 2.61 [1.25-3.97]; srsf2P95H + ENU 7.58 [5.27-9.88] [p<0.001]) (Figure 1B and C). These data are consistent with our previous findings showing that aberrantly spliced transcripts induced by the srsf2P95H mutation in CMML CD34+ cells were highly enriched in pathways that are involved in the repair of DNA damage, including the mismatch repair and base excision repair pathways, which represent the main system involved in the repair of damage induced by alkylating agents.
Collectively, our results suggest a model in which HSCs carrying CHIP-related mutations are deficient in repair systems, survive promutagenic genomic insults induced by chemotherapy, tolerate DNA damage accumulation, and acquire secondary alterations, which lead to malignant transformation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal