Abstract
Background
Patients diagnosed with Philadelphia-negative myeloproliferative neoplasms (MPNs) have been reported to have an elevated risk of thromboembolism. Although some data also indicate a higher risk of bleeding in this population, no study has yet compared the risk of bleeding in MPN patients to a control population. Therefore, using population-based data from Swedish registers, we evaluated the risk of bleeding in patients with MPN compared to matched controls.
Patients and Methods
All patients diagnosed with MPN between 1987 and 2009, and reported to the Swedish Cancer Register and the Swedish National Inpatient Register were included in the study. Four controls for each patient were randomly selected from the Register of Total Population and matched based on age, and sex. The outcome-data were collected from the Inpatient Register, the Outpatient Register and the Cause of Death Register. Major bleedings were defined as bleedings requiring hospital admission and/or with a fatal outcome. All other bleedings were considered minor bleedings.Cox regression models were used to obtain proportional hazard ratios with 95% confidence intervals (CIs), whereas flexible parametric survival models were used to assess time-varying HRs when there was no evidence of proportional hazards.Patients were followed until the time of event or to censoring due to death, immigration, or the end of follow-up 31st December 2009, whichever occurred first.
Results
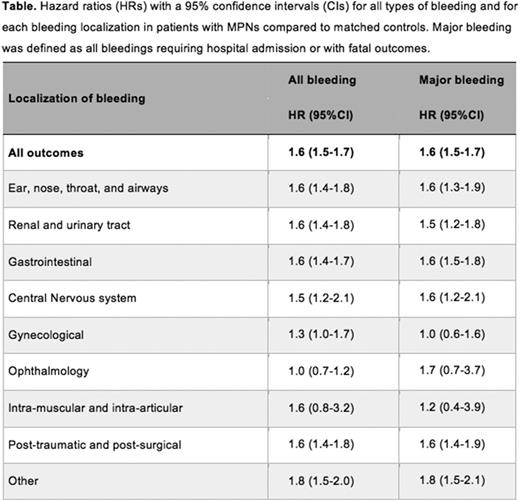
A total of 9,429 patients and 35,820 matched controls were included in this study. The median age at diagnosis was 72 years and 54% of patients were women. Essential thrombocythemia and polycythemiaverawere the most frequent subtypes, representing 3,462 and 3,001 patients, respectively, whereas 1,488 patients had primary myelofibrosis and 1,478 patients were diagnosed as MPN unclassifiable. The overall HR of bleeding for MPN patients was 1.6 (95% CI 1.5-1.7, p-value<0.001), compared tomatchedcontrols (Table). When analyzing HRs of bleeding during follow-up time after MPN diagnosis, the HR wasat 3 months 2.6 (95% CI 2.3-2.8), at 1 year 1.8 (95% CI 1.6-1.9), and at 5 years 1.3 (95% CI 1.3-1.4). Regarding the MPNs subtypes, patients with primary myelofibrosis had the highest risk of bleeding with a HR of 2.4 (95% CI 2.1-2.7), followed by patients with essential thrombocythemia (HR 1.6, 95% CI 1.4-1.7), MPN unclassifiable (HR 1.8 95% CI 1.6-2.0) and polycythemia vera (HR 1.4 95% CI 1.3-1.5).The overall HR of major bleeding was 1.6 (95% CI 1.5-1.7, Table) and the HRs at 3 months, 1 year and 5 years were2.6 (95% CI 2.3-3.0), 1.7 (95% CI 1.6-1.9), and 1.3 (95% CI 1.3-1.4), respectively. When analyzing the different localizations of bleedings, the highest risk elevation in the MPN population was observed for bleedings from the ear-nose-throat tract (HR 1.6, 95% CI 1.4-1.8), kidney and urinary tract (HR 1.6, 95% CI 1.4-1.8), gastrointestinal tract (HR 1.6, 95% CI 1.4-1.7), and central nervous system (HR 1.6, 95% CI 1.2-2.1) (Table).
Conclusions
In this large population-based study we found that patients withMPN, especially primary myelofibrosis, hada higher risk of bleeding compared to the control population. MPN patients were particularly prone to contract bleedings in the ear-nose-throat tract, kidney and urinary tract, gastrointestinal tract, and the central nervous system. Interestingly, the overall risk of bleeding in our cohort was highest shortly after diagnosis. This could partially be dueto the secondary coagulopathies sometimes associated with initially untreated MPNs, aspirin used for primary thrombosis prophylaxis as well as anticoagulation treatment following thrombotic events. From a clinical point of view, these findings underline the importance of keeping a vigilant watch on bleedings early after diagnosis and point to the need to evaluate the global bleeding risk for each patient.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal