Abstract
Introduction: APL, while highly curable in the modern era, remains a therapeutic challenge in the high-risk subset, with high rates of early mortality and relapse compared to non-high-risk APL. Recent evidence has confirmed excellent outcomes in non- high-risk APL with the combination of ATO and ATRA, and a previous pilot study had indicated the efficacy of a combination of ATO + ATRA + GO in high-risk APL. The North American Leukemia Intergroup designed a larger phase 2 study to confirm the efficacy and safety of this combination in high-risk APL.
Primary Objectives: 1) assessment of 3-year event-free survival (EFS); 2) assessment of early (6 week) death rate.
Methods: Adult patients with newly diagnosed high-risk APL (WBC ≥10k/uL) were eligible. Induction therapy consisted of: ATRA (45 mg/m2/day), beginning on day 1; ATO 0.15 mg/kg/day, beginning on day 10; GO 9 mg/m2on day 1. ATRA and ATO were continued until remission achieved. Patients in remission went on to receive consolidation with ATO x 2 cycles, followed by ATRA + daunorubicin x 2 cycles, followed by GO x 2 cycles. Subsequent maintenance therapy consisted of ATRA + 6-mercaptupurine + methotrexate for up to 1 year.
Results: Between 2008 and 2013, 73 registered patients began protocol treatment. Median age was 46.5 years, with 52% females and 48% males. Sixty-two (85%) patients completed induction therapy as planned, and 50 patients (68%) completed all planned consolidation.
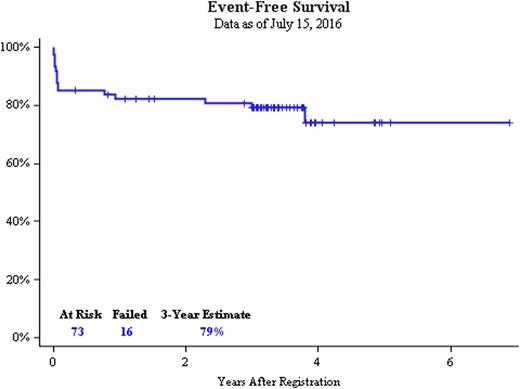
Sixty-two patients (85%) achieved a documented complete response (CR). Non-responses were attributable to lack of response assessment (n=10), most commonly related to death. One patient had resistant disease. With a median follow-up of 3.3 years, the Kaplan Meier 3-year EFS estimate was 79% (95% CI 68% - 87%), which was significantly improved compared to the protocol-defined historical rate of 50% (p<0.001). The 3-year overall survival estimate was 88% (95% CI 78% to 93%). The 3-year relapse-free survival estimate was 93% (95% CI 82% to 97%). At data cut-off, there was 1 documented death during remission and no reported cases of relapse.
Eight of 73 patients (11%) died within 6 weeks of treatment initiation (95% confidence interval 5-20%), at a median time of 11 days (range 3-31), which was significantly lower than the protocol defined null rate of 30% (p<0.001). The most common treatment-emergent grade 3-4 adverse events during induction included: febrile neutropenia (34%), AST/ALT elevation (12%), hypoxia/differentiation syndrome (11%), hyperglycemia (11%), headache (11%), prolonged QTc (11%).
Summary: the combination of ATO + ATRA + GO was safe and effective in patients with high-risk APL, producing highly durable responses, low rates of early mortality, and comparing favorably with chemotherapy-based induction regimens in this setting. Although GO is not currently commercially available in the US, this regimen should be considered as a therapeutic option for patients with high-risk APL in the future.
Support: NIH/NCI grants CA180888, CA180819, CA180820, CA180821, and in part by Wyeth Pharmaceuticals (Pfizer, Inc.)
Komrokji:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Speakers Bureau; Incyte: Consultancy. Coutre:Gilead Sciences: Consultancy, Research Funding. DeAngelo:Amgen: Consultancy; Ariad: Consultancy; Baxter: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Incyte: Consultancy; Celgene: Consultancy. Othus:Glycomimetics: Consultancy; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal