Abstract
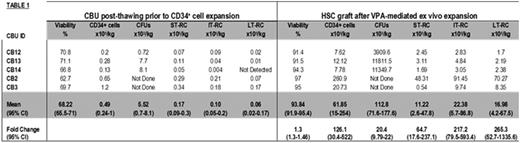
Umbilical CB is an established source of HSC for allogeneic transplantation in patients lacking HLA-matched donors. The major limitation of CB transplantation is the relatively low number HSCs within a single CB unit (CBU) resulting in delayed engraftment, infections and ultimately increased mortality. Because of this, double CBU transplantation has been used to reach the required cell doses but this approach has not led to an improved overall outcome and often results in an increased rate of GVHD. These findings have prompted the development of ex vivo expansion strategies to increase the number of HSCs, so that a single CBU can be transplanted. Most of these techniques, however, result in enrichment of short term marrow repopulating cells (ST-RC) at the expense of long term (LT)-RC which may impact durable long term engraftment. In addition, they require 2-3 weeks of culture which complicates the timing of transplant and increases the risk of contamination. Our laboratory has developed a novel approach to expand the numbers of functional HSCs, by transiently influencing the epigenetic determinants of HSCs self-renewal. A 7-day treatment of CB CD34+ cells with valproic acid (VPA) results in a dramatic increase in the number of HSCs capable of durable hematopoietic engraftment in animal model recipients (Chaurasia et al. JCI, 2014). Here we report the pre-clinical development of a VPA-expanded HSC product for utilization in the treatment of patients with hematological malignancies. In the place of using freshly collected CBU as starting sources of CD34+ cells, we validated, optimized and scaled-up the expansion procedure utilizing cryopreserved CBU procured from FDA-licensed Cord Blood Banks and clinically relevant GMP reagents and materials. CBUs from 5 different donors were subjected to thawing followed by positive CD34+ cell selection using a Miltenyi CliniMACS Prodigy®. The total number of nucleated cells (TNC), CD34+ cells, viability, clonogenic potential (i.e. CFU number) and the frequency of various HSC sub-classes were determined post-thawing after which each CBU was subjected to CD34+ cell selection. CD34+ cells counts varied between 1.6 and 13.6x106 (mean of 4.5x106/CBU) and had a purity ranging from 69.2-82.8%. CD34+ cells were treated with cytokines for 16-18h, followed by addition of VPA and ex vivo expansion for 7 days. The generated cell product was characterized phenotypically and functionally and the results were compared to the unmanipulated CBU (uCBU) (Table 1). First, the expanded grafts had greater than 90% viability (range 91.4 to 97%) as compared to 68.2% in the uCBUs after thawing. The average number of CD34+ cells generated was 494.8x106 CD34+ cells (i.e. 126-fold greater than uCBU) which is the equivalent of 61.8x105/Kg/ body weight from a single CBU for an 80 kg individual. The fraction of CD34+ cells, which represented over 60% of the expanded graft, was further assessed for the presence ST-RC, intermediate-term (IT)-RC and LT-RC defined phenotypically as CD34+/CD45RA-/CD90-/CD49f-, CD34+/CD45RA-/CD90+/CD49f-, and CD34+/CD45RA-/CD90+/CD49f+, respectively. The average numbers of each of these HSC sub-classes per expanded CBU were 64, 217 and 265 fold higher than their respective numbers found in the uCBUs. Notably, the expanded grafts contained the equivalent of 22.38x105 IT-RC/kg and 16.98x105 LT-RC/kg where as uCBUs contained only 0.1x105 IT-RC/kg and 0.06x105 LT-RC/kg. Considering the ability of these HSC sub-classes to contribute to intermediate and long term hematopoietic engraftment, their presence in such high number gives the VPA-expanded grafts improved potential to lead to durable hematopoietic and immune reconstitution after transplantation. In addition, the expanded graft has a phenotype which would also be anticipated to lead to rapid hematopoietic recovery since lineage committed precursors (i.e. CD33+, CD15+, CD235a+ and CD41+ cells) represented 35-45% of its composition. Finally, as compared to uCBUs, the expanded HSC product contained 20 times more assayable CFUs consisting predominately of CFU-GEMM which are capable of contributing to multilineage engraftment. In summary, we report the generation of an ex vivo expanded CB HSCs product highly enriched in primitive HSCs sub-classes and which is currently being developed for a Phase I clinical trial for allogeneic CB transplantation in patients with hematological malignancies.
Bhardwaj:Parker Institute of Cancer Immunotherapy: Membership on an entity's Board of Directors or advisory committees; Checkpoint Sciences: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal