Abstract
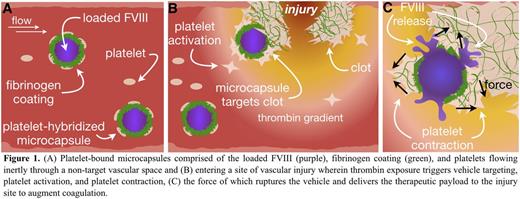
Introduction: Treating bleeds in hemophilia A patients with inhibitory antibodies remains extremely challenging as the patient antibodies render the most commonly used treatment, systemically delivered Factor VIII (FVIII), ineffective. As such, patients often must undergo costly immune tolerance induction therapy with marginal success or use bypassing agents with higher risks of thrombosis. Theoretically, encapsulating FVIII in a microcapsule may protect it from inhibitory antibodies until its release. To that end, we have developed FVIII loaded microcapsules that display fibrinogen on their exterior, allowing them to be adherent to patient platelets upon intravenous administration (Figure 1A). These microcapsules circulate inertly until they reach vascular injury sites whereupon localized agonists activate the adherent platelets (Figure 1B). The contractile force of the adherent and nearby platelets will then mechanically rupture the microcapsule walls and release FVIII (Figure 1C), which will be incorporated into the nascent clot before inhibitory antibodies can take effect. Leveraging the biomechanical behavior of activated platelets as both the sensor and actuator is a paradigm-shifting methodology to accomplish targeted, controlled FVIII delivery to evade inhibitory antibodies and augment hemostasis.
Results and Discussion:Microcapsules were fabricated via layer-by-layer deposition of alternatingly charged polyelectrolytes (PE), poly-L-lysine and poly-L-glutamic acid, onto calcium carbonate cores. Fibrinogen was incorporated into the final PE layer to facilitate binding with platelets. A double core strategy was employed containing a FVIII inner core and a dextran outer core. After the desired number of layers was deposited, the core was chelated out, resulting in microcapsules with a dextran layer separating the PE wall from loaded FVIII (encapsulation efficiency = 45±4%).
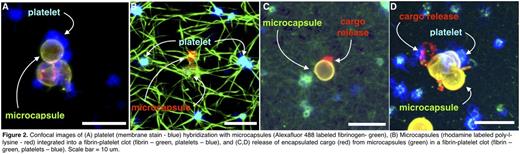
Static in vitro experiments showed that platelets adhered to the microcapsule surface (Figure 2A) and microcapsules incorporated into fibrin networks upon platelet activation (Figure 2B). Furthermore, once integrated into the fibrin network, microcapsules rupture during clot contraction, resulting in platelet-actuated delivery in target environments (Figure 2C,D). Microcapsule rupture occurs only in the vicinity of contracting platelets, ensuring drug delivery occurs only at sites of active clot formation.
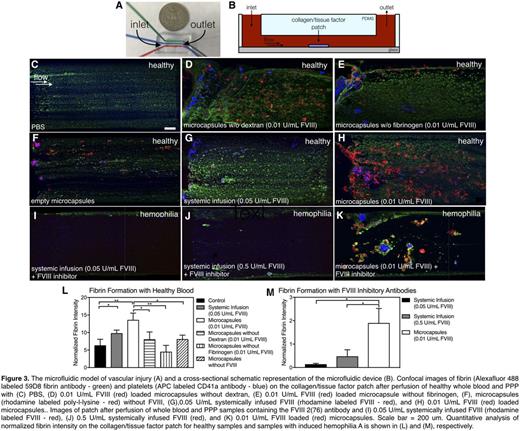
Perfusion of recalcified whole blood and platelet poor plasma into in vitro microfluidic models of vascular injury (Figure 3A,B) comprising a collagen/tissue factor patch were used to evaluate the efficacy of FVIII delivered either systemically or in microcapsules. FVIII efficacy was quantitatively evaluated by comparing fibrin fluorescence intensity on the patch, which was normalized to platelet number as it was found platelet number influenced fibrin formation. No significant difference in fibrin formation was seen compared to the PBS control (Figure 3C) using microcapsules without dextran, without fibrinogen, or without loaded FVIII (Figures 3D-F). However, compared with standard systemic infusion of 0.05 U/mL FVIII (Figure 3G), microcapsules loaded with 0.01 U/mL FVIII (Figure 3H) produced 4x as much fibrin (Figure 3L). To mimic hemophilia A blood with inhibitors, the FVIII inhibitory antibody 2(76) was introduced into healthy blood samples. Efficacy of microcapsules loaded with 0.01 U/mL FVIII was compared to systemically infused FVIII at 0.05 and 0.5 U/mL, clinically relevant low and high dosages (Figure 3I-K). Here, significantly more fibrin was produced in samples with microcapsules than either condition with systemic FVIII infusion (p<0.05) (Figure 4M). This increased efficacy is likely due to the microcapsule shielding effect on FVIII, preventing exposure to inhibitory antibodies
Conclusions: These microcapsules use platelets from the patient to target and deliver encapsulated FVIII - a new paradigm in targeted drug delivery. In vitro experiments show this technology increases FVIII efficacy for hemophilia A patients with inhibitors due to shielding from neutralizing antibodies. Current work evaluating localized thrombin generation due to the FVIII loaded microcapsules and the effect of platelet contraction force via pharmacologic agents such as blebbistatin, ROCK, and myosin inhibitors are ongoing.
Meeks:Biogen: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Bayer Healthcare: Membership on an entity's Board of Directors or advisory committees; Grifols: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees. Lam:Sanguina, LLC: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal