Abstract
Background
The activated B-cell (ABC) type of diffuse large B-cell lymphoma (DLBCL) is clinically more aggressive than the germinal center (GCB) type. The gene expression profile of ABC DLBCL resembles that of mature B cells upon stimulation via their B-cell receptor (BCR). In app. 10% of ABC DLBCL cases, this signature can be explained by gain-of-function mutations in CARD11. Recent evidence suggests that autoantigen recognition by the BCR sustains the viability of ABC DLBCL in vitro (Young, PNAS 2015), although the functional relationship to CARD11 mutations remains unresolved. Antigen-independent, constitutively active BCR signaling is a fundamental oncogenic mechanism of CLL (Dühren-von Minden, Nature 2012). We therefore explored the hypothesis that autonomous BCR signaling akin to CLL could be operative in ABC DLBCL and would represent an alternative but functionally complementary oncogenic mechanism to activating CARD11 mutations in ABC DLBCL lymphomagenesis.
Methods
BCR transcripts were identified from archived fresh-frozen biopsies of 14 histologically confirmed, CD10-negative, IgM-expressing DLBCL by ARTISAN PCR, a novel anchored RT-PCR for unbiased isolation of full-length BCR transcripts facilitated by template switching (Koning, BJH 2016). Clonal full-length BCR were identified by single molecule sequencing on the PacBio platform. All biopsies were screened for mutations in CARD11 and CD79 by Sanger sequencing. To test for autonomous BCR signaling activity, murine triple KO (TKO) cells were transduced with functional DLBCL BCR (Dühren-von Minden, Nature 2012). TKO cells lack the rag2 and lambda5 genes and are therefore developmentally arrested at the pre-B-cell stage. In addition, TKO cells have their wild-type SLP65 adaptor replaced with a tamoxifen-dependent SLP65 variant. When transduced with a functional BCR, autonomous or antigen-induced BCR signaling can be measured upon restoring functionality of the BCR signalling cascade with tamoxifen as calcium flux by flow cytometry and robust cellular proliferation.
Results
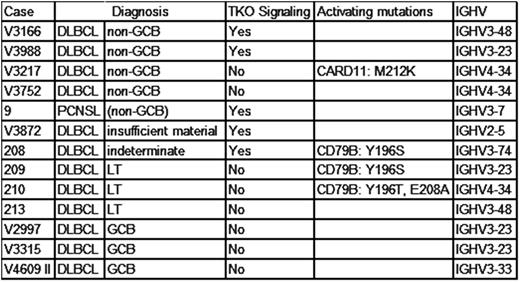
Re-examination by immunohistochemistry for CD10, PAX5, MUM1, and Bcl-6 was possible in 12 cases and identified three cases as GCB-type DLBCL. Nine cases were classified as non-GCB DLBCL, including one primary CNS DLBCL and three leg-type primary cutaneous DLBCL (LT DLBCL). One non-GCB DLBCL was found to carry a potentially activating CARD11 M212K mutation (Table).
BCR of the three GCB DLBCL served as controls and lacked an autonomous BCR signal. In contrast, TKO cells transduced with five of the seven remaining DLBCL BCR (71%) showed robust calcium flux and proliferation upon activation of SLP65 by tamoxifen without additional BCR crosslinking. In accordance with our hypotheses, the CARD11-mutated non-GCB DLBCL case lacked autonomous BCR signaling. This case and the second, non-GCB DLBCL case lacking autonomous BR activity that carried wild-type CARD11 both expressed a BCR with the IGHV4-34 allele. Unexpectedly, the three LT DLBCL also did not exhibit autonomous BCR signaling.
Conclusion
Our data demonstrate autonomous BCR activity in a dominant fraction of non-GCB DLBCL. In striking similarity to CLL, these results point to an oncogenic role of a structurally normal BCR in non-GCB DLBCL that does not require binding of external autoantigens. Consistent with our hypotheses, antigen-independent signaling of a structurally normal BCR signaling cascade is a compelling candidate alternative mechanism to activating mutations in signal-transducing components of the BCR pathway. Both mechanisms may therefore induce the characteristic gene expression signature of ABC DLBCL. In addition, this finding readily explains the higher likelihood of ABC DLBCL with wild-type CD79 and CARD11 to respond to BCR signaling inhibition by BTK inhibitors (Wilson, Nat Med 2015). In contrast, LT DLBCL, which otherwise resembles ABC DLBCL, does not exhibit autonomous BCR signaling, suggesting yet another oncogenic mechanism that may explain its dismal prognosis. Finally, the non-GCB DLBCL lacking both autonomous BCR signaling and a potentially activating CARD11 mutation expressed the inherently autoreactive IGHV4-34 allele, opening the possibility that strong autoantigen stimulation might substitute for autonomous BCR signaling activity, thereby offering an alternative explanation for the BCR signaling pathway addiction in IGHV4-34-expressing cases.
Vermeer:Innate Pharma: Other: Investigator in a clinical trial.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal