Abstract
Introduction
The need to optimize clinical evaluation of new drugs stimulates researchers and regulatory bodies to consider novel endpoints that facilitate assessment of the efficacy of a new drug earlier in time than do traditional endpoints. To be a useful marker of efficacy, an early endpoint must be an accurate surrogate for the true clinical endpoint.
Minimal residual disease (MRD) is a strong prognostic factor for Event Free Survival (EFS) in children with newly diagnosed acute lymphoblastic leukemia (ALL) and is used routinely to assess treatment response and stratify treatment intensity. However, it is not known whether or not early MRD response is an accurate surrogate endpoint for EFS in evaluating the efficacy of treatment interventions.
This study addresses for the first time in childhood ALL the formal validation of surrogacy of MRD levels at the end of induction treatment by a meta-analytic approach on individual data from two large phase III trials with a randomized question on type of steroids in induction (dexamethasone 10mg/m2/day vs prednisone 60mg/m2/day).
Material and Methods
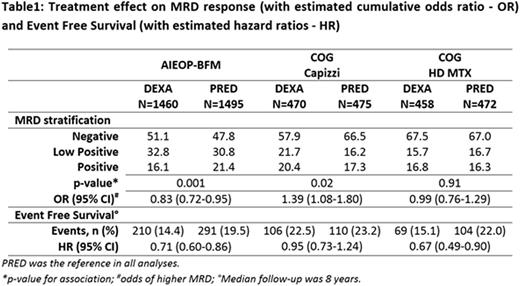
We performed a meta-analysis of individual data of 2955 B-ALL patients from AIEOP-BFM-ALL2000 (NCT00613457, NCT00430118), and 945 and 930 high risk B-ALL patients randomized for steroids after being allocated either to Capizzi or High-dose Methotrexate in COG AALL0232 (NCT00075725; separately considered due to the significant quantitative interaction between Methotrexate regimens and type of steroid). The trials included evaluation of MRD at day +33 (PCR-MRD) and +29 (flow-cytometry MRD), respectively, with a sensitivity of at least 10-4. The three categories MRD level (negative, low positive i.e. <5x10-4 and positive ≥5x10-4), was assessed as surrogate for the EFS endpoint (time to event defined as resistance at the end of induction, relapse, death in remission, second malignancy).
A two-level modelling approach was used to estimate the association between MRD and EFS and between the treatment effect on MRD (proportional odds model) and on EFS (proportional hazard model). The quality of the surrogate at the individual level was assessed on the basis of the bivariate Plackett copula model, with a parameter representing the global odds ratio. The quality of the surrogate at the trial level was assessed on the basis of the coefficient of determination R2trial from a linear regression through the estimated treatment effects.
Results
The main results on MRD and EFS by trial and treatment are in table 1.
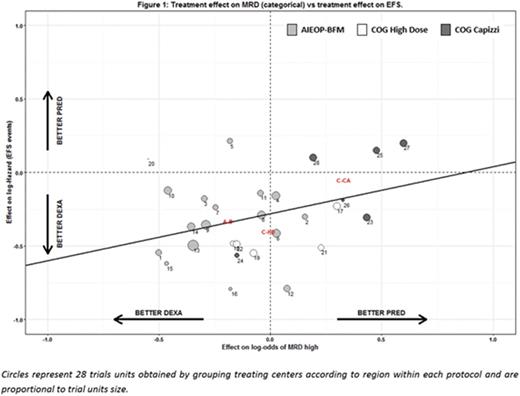
The meta-analytic approach shows that MRD at the end of induction is a poor surrogate for treatment effect on EFS (Figure 1) at the trial level, with R2trial=0.09 (95% CI: 0-0.29), while, at the individual level, it shows a considerable prognostic association with EFS, after adjusting for treatment, with a 3.9 odds ratio of failure for patients with higher compared to lower MRD levels (95% CI: 3.4-4.4). Additional sensitivity analyses on relevant subgroups generally confirmed the previous findings both at the trial and patient level association.
Conclusions
Using a meta-analytic approach, we found that MRD, in 3 categories defined according to standard cut-points, is a poor surrogate for EFS at the trial level, thus indicating that the effect of the randomized steroids (dexamethasone vs. prednisone in induction) on the MRD level at the end of induction does not reliably predict the effect of the intervention on EFS. In contrast, the analysis shows a strong and highly significant association between end induction MRD level and EFS time for individual patients, regardless of treatment, confirming the prognostic effect of early MRD response on clinical outcome.
This study shows, for the first time, the limitation of a strong prognostic factor in being a surrogate in the context of front line ALL treatments. The impact of type of steroid on MRD distribution at the end of induction is relatively limited and subsequent treatment complexity and intensity, partly tailored on MRD itself as a key criterion used to modulate the intensity of post-induction therapy, may dilute a potential surrogacy. These data suggest that clinicians and regulatory bodies should be cautious in using early MRD response in the context of complex multiagent therapy as an early surrogate endpoint to evaluate the effect of a randomized treatment intervention on long-term EFS.
Moricke:JazzPharma: Honoraria, Other: financial support of travel costs. Biondi:Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; BMS: Membership on an entity's Board of Directors or advisory committees; Cellgene: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal