Abstract

Background: Minimal residual disease (MRD) assessment after initial therapy is integral to modern risk stratification in both precursor B and T lineage acute lymphoblastic leukemia (B-ALL and T-ALL). While MRD is used to determine depth of remission, remission is still defined, both in clinical practice and clinical trials, according to morphological assessment. We aimed to determine the outcomes of children, adolescents and young adults with discordant assessments of remission by morphology vs. by MRD, and in doing so, the extent to which morphologic assessment of remission contributes to risk assessment in this population.
Methods: We identified a cohort of patients age 1-30.99 years enrolled on frontline COG trials for B-ALL [standard risk (SR): AALL0331; high risk (HR) AALL0232] and T-ALL (AALL0434) that underwent bone marrow assessment of remission at the end of induction therapy (Day 29). Morphologic response was assessed by local centers and was categorized according to traditional criteria: M1 (<5% leukemic blasts - remission) vs. M2 (5-25%) vs. M3 (>25%). MRD was measured by flow cytometry at one of two central laboratories. We determined predictors of MRD discordance and compared event free survival (EFS) between those with discordant vs. concordant morphology/MRD remission assessments.
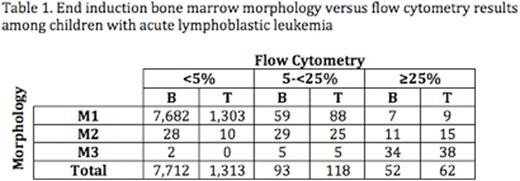
Results: Day 29 remission assessments and central MRD data were available on 9,350 patients, 7,857 (84%) with B-ALL (AALL0331: N=5049; AALL0232: N=2808) and 1,493 (16%) with T-ALL. Table 1 shows the distribution of end induction marrow morphology vs. flow cytometry results. Few patients with M2/M3 marrows had discordant low MRD values. For example, of 84 patients with M3 morphology, only 2 (2.4%) had MRD <5%. Of 202 patients with M2/M3 morphology, 23 (11.4%) had MRD<1% and 9 (4.5%) had MRD<0.1%. Subsequent analyses of discordance were thus restricted to patients with M1 morphology but flow cytometry consistent with failure to achieve remission (MRD>=5%). Using this definition, discordance was uncommon among subjects with B-ALL (66/7,748; 0.9%) but significantly more common in T-ALL (97/1,400; 6.9%; p<0.0001). Among subjects with B-ALL and M1 morphology, significant predictors of discordance (MRD>=5%) in multivariable regression included variables traditionally associated with poor response: age >=10 years [odds ratio (OR)=1.7, 95th percentile confidence interval (CI) 1.1-2.8; p=0.03), presenting white blood cell count >=50,000/microliter (OR=2.1, CI 1.3-3.6; p=0.004), and unfavorable compared to favorable cytogenetics (OR=31, CI 8.9-109; p<0.0001). In B-ALL, subjects with end induction M1 morphology but discordant MRD (>=5%) had modestly superior 5-year EFS when compared to those with M2 morphology and MRD >=5% (33.1%±6.2% vs. 22.0%±6.9%; p=0.03), but EFS was significantly inferior to those with M1 morphology and concordant MRD (<5%) (33.1%±6.2% vs. 86.8%±0.4%; p<0.0001) (Figure 1). In T-ALL, the 5-year EFS of subjects with M1 morphology/discordant MRD was not significantly different from those with M2 morphology and MRD >=5% (80.3%±7.3% vs. 62.7%±13.5%; p=0.13); outcomes of both groups were superior to their equivalents with B-ALL, in keeping with known slower disease clearance kinetics in T-ALL.
Conclusions: Patients in morphologically defined remission but with MRD >=5% have outcomes similar to those who fail to achieve morphological remission. These results suggest that, in addition to measuring depth of remission, MRD should replace morphology in defining remission in subjects with ALL, with consequent implications for risk stratification, treatment assignment and eligibility for experimental agents.
Loh:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding. Borowitz:HTG Molecular: Consultancy; Bristol-Myers Squibb: Research Funding; MedImmune: Research Funding; BD Biosciences: Research Funding. Wood:Juno: Other: Laboratory Services Agreement; Pfizer: Honoraria, Other: Laboratory Services Agreement; Amgen: Honoraria, Other: Laboratory Services Agreement; Seattle Genetics: Honoraria, Other: Laboratory Services Agreement.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal