Abstract
Background: Allogeneic hematopoietic stem cell transplantation (HCT) is the only potentially curative therapy for myelodysplastic syndrome (MDS). However, mortality after HCT for MDS is high, with deaths attributable to both relapsed disease and transplant-related complications. The genetic lesions that drive MDS pathogenesis have the potential to predict the outcome of patients following HCT.
Methods: We performed targeted sequencing of 127 genes on pre-HCT blood samples from 1514 patients who received allogeneic HCT for MDS between 2005 and 2014. All MDS patients enrolled in the Center for International Blood and Marrow Transplant Research (CIBMTR) Repository were considered for inclusion. Patients were excluded only if blasts were >/= 20% or if they had diagnosis of CMML or MDS/MPN overlap. The median age of patients was 59 years (range 0.4 - 77 years), including 241 (16%) < 40 years of age. Donors were 8/8 HLA-matched unrelated donors (n = 862, 57%), HLA-identical siblings (n = 181, 12%), < 8/8 HLA-matched unrelated donors (n = 296, 20%), or umbilical cord blood (n = 174, 11%).
Results:TP53 mutations were independently associated with poor overall survival (HR 1.71, 95% CI 1.45-2.02, p < 0.0001) and a shorter time to relapse (HR 2.34, 1.85-3.00, p < 0.0001). In a multivariable model the adverse prognostic significance of TP53 mutations was independent of recipient age, performance status, hematologic status at time of HCT, bone marrow blast count, and karyotype. RAS pathway mutations were independently associated with a shorter time to relapse (HR 1.73, 1.34-2.23, p < 0.0001) and JAK2 V617F mutations were associated with higher transplant-related mortality (TRM) (HR 2.13, 1.38-3.27, p = 0.0006).
Patients with therapy-related MDS (t-MDS) comprised 21% of the cohort (n = 311) and had significantly worse survival than patients with primary MDS (HR 1.4, 1.2-1.6, p = 0.0002). Only TP53 (OR 3.8, p <0.0001) and the p53 regulator PPM1D (OR 4.8, p < 0.0001) were selectively mutated in t-MDS. Overall survival was inferior in t-MDS patients with TP53 mutations (HR 1.7, 1.3-2.3, p = 0.0004), but similar in t-MDS patients without TP53 mutations compared to patients with primary MDS (HR 1.1, 0.9-1.4, p = 0.37).
In univariate analysis, young recipient age was powerfully associated with good post-transplant survival. However, we identified a group of adult patients under 40 years old with compound heterozygous mutations in the Shwachman-Diamond Syndrome (SDS)-associated SBDS gene that had remarkably poor prognosis and no long-term survivors (HR 6.0, 1.6-23.0, p = 0.009). All patients with biallelic SBDS mutations had somatic TP53 mutations, suggesting a biological synergy between SBDS and TP53 mutations in clonal MDS transformation of genetically-defined SDS.
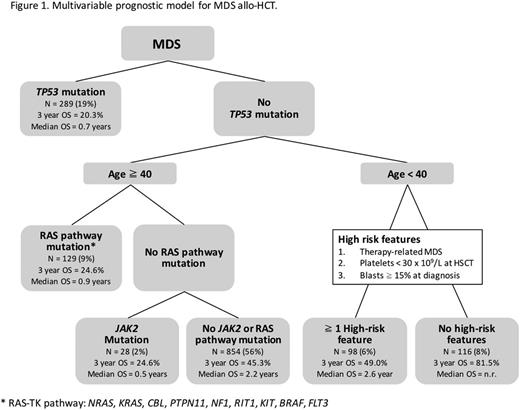
Finally, we used recursive partitioning to identify 6 distinct MDS subgroups based on the association between clinical and genetic variables and post-HCT survival, then evaluated each subgroup to identify links to relapse or TRM (Figure 1). Consistent with our multivariable model, the presence of TP53 mutations was the most important prognostic variable, with mortality driven by relapse and not influenced by conditioning intensity. In patients age 40 and older without TP53 mutations, two subgroups had poor outcomes: those with RAS pathway mutations, with median OS of 11 months and high risk of relapse (p = 0.001), and those with JAK2 mutations, with median OS of 6 months and high risk of TRM (p < 0.001). Among patients under 40 years old without TP53 mutation, we identified two subgroups based on evaluation of three high-risk clinical features (t-MDS, platelets < 30 x 109/L at HCT, or bone marrow blasts at diagnosis >/= 15%). Patients < 40 years old with at least one high-risk feature had 3 year OS of 49.0%, associated with high TRM (p < 0.001), while those with no high-risk features had 3 year OS of 81.4%.
Conclusions: We found that TP53 mutations are the most important predictor of prognosis in allo-HCT for MDS, independent of other clinical and genetic variables, and that the adverse outcome in TP53 mutated MDS is not influenced by conditioning intensity, donor type, or graft source. TP53 mutations account for the characteristically poor outcomes of clinically distinct subgroups, including t-MDS and genetically-defined SDS. We present a multivariable model that identifies 6 distinct MDS subgroups with differential post-HCT survival and differences in relapse and TRM.
Lindsley:Takeda Pharmaceuticals: Consultancy; MedImmune: Research Funding. Mar:H3 Biomedicine: Other: Spouse's employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal