Abstract
Background
Spleen tyrosine kinase (SYK), a nonreceptor kinase with a key role in B-cell receptor signaling-driven tumorigenesis, is a viable target for inhibition in B-cell malignancies. SYK is also a key regulator of FMS-like tyrosine kinase 3 (FLT-3), which is mutated in 30% of pts with acute myeloid leukemia (AML; Puissant et al. Cancer Cell 2014;25:226-42). TAK-659, a selective, reversible, potent SYK/FLT-3 inhibitor demonstrated inhibition of SYK activity and cell proliferation in vitro and inhibited dose-dependent tumor growth in vivo in lymphoma xenograft models. The TAK-659 maximum tolerated dose (MTD) is 100 mg daily (QD) based on phase 1 data (NCT02000934; Petrich et al. Blood 2015;123:2693). Here we present updated safety and efficacy data from the expansion phase of this study.
Methods
Adult pts with advanced solid tumors/lymphoma, with no standard effective treatment available, received oral TAK-659 QD in 28-d cycles: 60-120 mg during dose escalation or 100 mg during dose expansion. AEs were assessed per NCI-CTCAE v4.03. Responses per RECIST v1.1 (solid tumors) or IWG modified criteria (lymphoma) were assessed between d22 and d29 (predose) of C2 (both phases), then during C4, C6, and every 3 cycles (escalation) or every even numbered cycle until C12, then every 4 cycles (expansion). During dose escalation, blood samples for plasma PK were collected predose and at multiple times postdose on d1 and d15 of C1; urine samples were collected on d15 of C1, 0-8 hrs postdose. Next-generation sequencing and NanoString analyses are planned to identify mutations correlating with clinical response and to classify lymphoma pts by cell of origin using Lymph2Cx signature.
Results
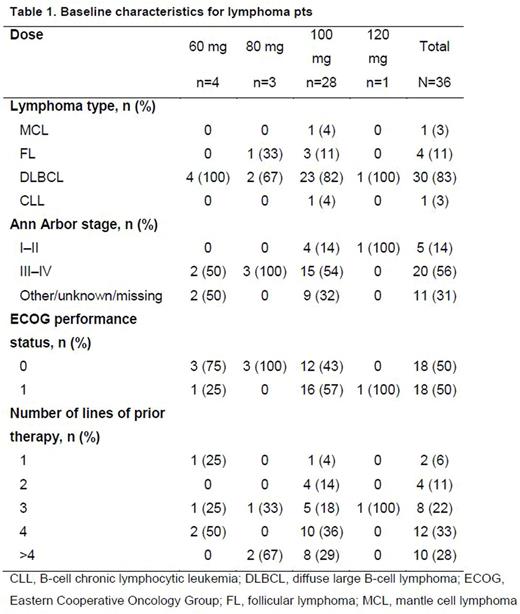
At data cut-off (April 27, 2016), 54 pts had received TAK-659. During dose escalation, 35 pts (18 solid tumor; 17 lymphoma [12 DLBCL; 4 FL, 1 MCL]) received TAK-659 QD at 4 dose levels: 60 mg (n=10), 80 mg (n=4), 100 mg (n=14), and 120 mg (n=7). During expansion, 19 lymphoma pts (18 DLBCL; 1 CLL) received TAK-659 QD at 100 mg (MTD). Median age for the overall population was 60.5 years (range, 23-82); 70% were male. Baseline characteristics for the 36 lymphoma pts are shown in Table 1. Of 30 DLBCL and 4 FL pts, 24 and 3, respectively, were response-evaluable as of June 9, 2016 (received ≥1 dose of TAK-659; had ≥1 post-treatment response assessment). By local assessment, 14 pts were de novo (7 GCB, 4 non-GCB, 3 unknown) and 10 were transformed (8 GCB, 2 non-GCB).
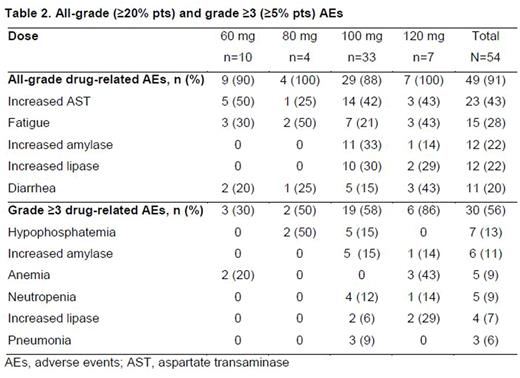
There were 5 dose-limiting toxicities (DLTs): at 120 mg, 2 pts had increased lipase levels (1 gr 3; 1 gr 4), 1 pt had gr 3 generalized edema, and 1 had gr 3 mucositis; 1 pt at 60 mg had gr 3 increased AST. DLTs related to laboratory changes were asymptomatic. LDH levels were increased from baseline in most pts (significance unknown). Drug-related AEs are shown in Table 2.
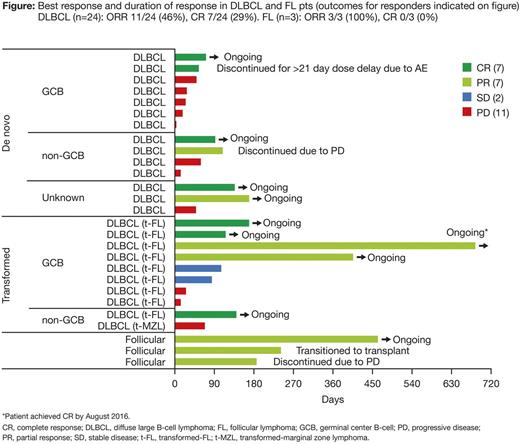
Drug-related serious AEs (per investigator) were reported for 17 (31%) pts; most common were pneumonia (5 [9%]), pyrexia (3 [6%]), and pneumonitis (2 [4%]). Dose reductions due to AEs occurred in 4 pts (3 from 100 to 80 mg; 1 from 120 to 60 mg). Nine pts discontinued due to AEs; 3 were considered related to TAK-659 (1 sepsis, 2 pneumonitis). There were 10 on-study deaths; 1 was considered possibly related to TAK-659 (sepsis). Preliminary plasma and urine PK data showed that TAK-659 was absorbed quickly (median Tmax 2-3 hrs), with moderate variability in steady-state exposures (40-50% CV for DN-AUCtau), mean peak/trough ratio of 3.2-4.2, and mean accumulation of 2.1- to 2.6-fold after 15 d QD dosing. Renal clearance (CLr) of unchanged drug accounted for 30-34% of apparent oral clearance, suggesting a CLr contribution of ≥30-34% to TAK-659 systemic clearance. Of 24 response-evaluable DLBCL pts, 11 (46%) achieved an objective response; (7 [29%] CR, 4 PR); all 3 response-evaluable FL pts achieved PR; 10/14 responders are ongoing on the study (Figure). Median treatment duration for responders was 138 days for DLBCL (range 54-688) and 237 days for FL (range 185-464). Responses were seen in both de novo and transformed DLBCL pts and appeared to be independent of cell-of-origin classification. No solid tumor pts achieved an objective response.
Conclusions
Oral TAK-659 has an acceptable PK and safety profile in pts with solid tumors or lymphoma, supporting continuous oral QD dosing. There are early signs of clinical activity in DLBCL and FL pts. Enrollment of pts with additional lymphoma subtypes is ongoing; safety and efficacy will be further investigated.
Kaplan:Janssen: Research Funding; Seattle Genetics: Research Funding. Gordon:Northwestern University: Patents & Royalties: Patent for gold nanoparticles pending. Infante:Millennium: Research Funding. Popat:Takeda: Honoraria. Madan:Onyx: Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Celgene: Speakers Bureau. Radford:Novartis: Honoraria, Speakers Bureau; Seattle Genetics: Honoraria, Speakers Bureau; GSK: Equity Ownership; Astra-Zeneca: Equity Ownership; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau. de Oteyza:Takeda: Research Funding. Zinzani:Abbvie: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Millennium: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Infinity: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Faucette:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Sheldon-Waniga:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Williams:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Stumpo:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Shou:Millennium Pharmaceuticals Inc., Cambridge, MA, USA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Bosch:Hoffman La Roche: Consultancy, Honoraria, Research Funding; Millennium: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal