Abstract
Adoptive immunotherapy with transplant donor derived virus specific T cells is an effective strategy for the treatment of CMV viremia and disease arising after an allogeneic hematopoietic stem cell (HSCT). This approach is not readily applicable if the donor is seronegative or not available to provide lymphocytes for in vitro expansion for CMV specific cytotoxic T lymphocytes (CMV-CTL) lines or if the CMV CTL lines derived from non-identical donors are restricted by non-shared HLA alleles. We and others have previously presented results indicating that treatment with in vitro expanded CMV-CTLs derived from an HLA partially matched third party donors can effectively treat CMV infections in the post-transplant setting. However, the patient population most likely to benefit from this approach has not been well defined.
Patients at especially high risk of succumbing to CMV infection include those who acquire resistance to antiviral therapy. We now present results of treatment with banked off-the-shelf third party CMVpp65 specific CTLs in patients with genetically defined mutations in the UL54 DNA polymerase and UL97 kinase CMV genes predicting resistance to antiviral agents. Fifteen recipients of HSCT with mutations defined prior to the start of cell therapy were treated. All 15 had mutations associated with resistance to ganciclovir. Overall 5 had resistance to ganciclovir, foscarnet and cidofovir, 5 had resistance to ganciclovir and foscarnet, one had resistance to ganciclovir and cidofovir and 4 had resistance to ganciclovir alone.
Third party CMV-CTLs were selected on the basis of HLA matching at high resolution at a minimum of 2/8 recipient alleles and HLA restriction of the T cells by one or more HLA alleles present in the patient. CMV-CTLS were selected from a bank of 132 lines generated under GMP conditions from normal HSCT donors specifically consented for use of their T cells in patients other than the designated transplant recipient. These 15 patients had a median age of 60.5 (7.4-70.1) years and started therapy with CMV-CTLs a median of 157 (70-564) days after reactivation of CMV. Four of these patients had CMV disease while 11 were treated for viremia alone. Prior therapy in this cohort included foscarnet (N=15) ganciclovir and/or valganciclovir (N=14) and cidofovir and/or brincidofovir (N=9). Each cycle of CMV-CTLs consisted of 3 weekly infusions of 1x106 T-cells/kg/infusion. These 15 patients received a median of 2 (1-3) cycles of CMV-CTLs. CMV-CTLs were well tolerated and there were limited toxicities. Six patients experienced AEs of which one grade 3 and one grade 4 AE were deemed possibly related to infusions with CMV-CTLs.
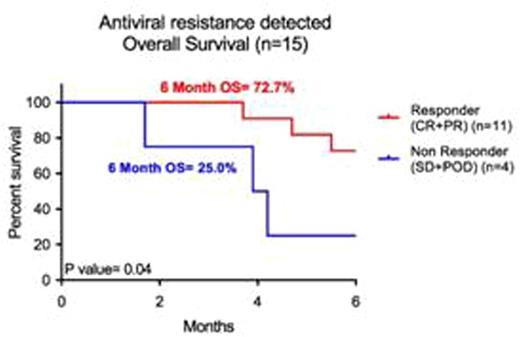
Overall 11/15 (73.3%) patients responded to CMV-CTL therapy including 2 patients with disease with 6 CRs and 5 PRs. Overall survival at 6 months in the 11 responders and 4 non-responders was 72.7% and 25.0% respectively. Within the 6 month follow-up one of the 11 responding patients died of CMV while 3 of the 4 non-responding patients died of CMV. These results indicate that third party donor derived "off-the-shelf" CMV-CTLs can effectively treat CMV viremia or disease in patients not responding to antiviral therapy with demonstrated genetic resistance to antiviral agents.
Doubrovina:Atara Biotherapeutics: Consultancy, Research Funding. Hasan:Atara Biotherapeutics: Consultancy, Research Funding. Koehne:Atara Biotherapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal