Abstract
Background:
Immune tolerance in solid organ transplantation is a continuing challenge because of the risks of acute and chronic rejection and severe adverse effects of extended exposure to immunosuppressive drugs (IsD). Therefore, the goal of transplant tolerance is to prevent both acute and chronic rejection without use of prolonged IsD. Owen [Science 1945] and Billingham [Nature 1953] demonstrated solid organ transplant tolerance by inducing stable donor:host chimerism with infusion of hematopoietic stem cells (HSCT). Subsequently, various animal studies and clinical trials demonstrated the ability ,of HSCT to induce solid organ transplant tolerance. Herein we present a case of an haplo-identical (HaploTx) HSC for the treatment of multiple myeloma (MM) followed thereafter by a renal transplant from the same donor. We discuss this case and results reported in the literature.
Case Report:
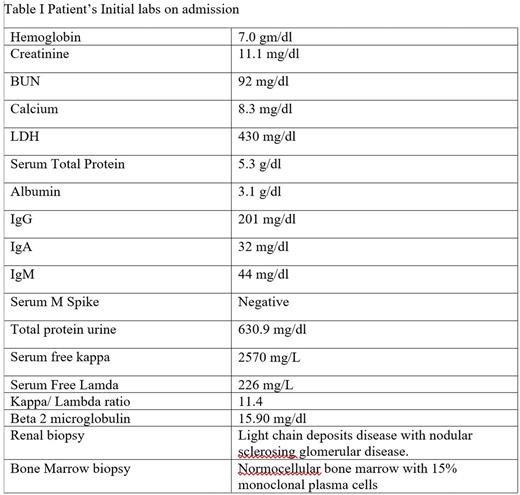
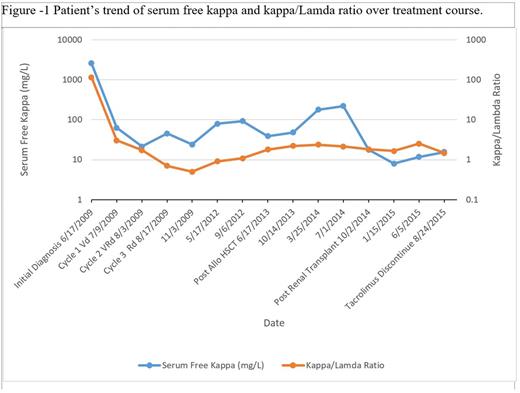
A 25-year-old male presented to the ER with complaints of fatigue, nausea, and intermittent vomiting for six months. Ultimately, he was diagnosed with kappa light chain multiple myeloma, International Staging System 3, with light chain cast nephropathy on renal biopsy (Table I). He initiated hemodialysis for worsening renal function and uncontrolled hypertension. He began chemotherapy with bortezomib, lenalidomide and dexamethasone (VRd). He responded well to chemotherapy achieving a partial remission. His free kappa light chains decreased from 2570 mg/L to 63 mg/L after 3 cycles of VRd. Considering his age, response to chemotherapy, and end stage renal disease requiring hemodialysis, the patient was offered an allogenic HSCT to be followed 1 year thereafter by a renal transplant from the same donor. Since he did not have any matched sibling donors, he underwent an HaploTx from his father after a conditioning regimen with fludarabine, cyclophosphamide and total body irradiation followed by infusion of bone marrow cells. The post-transplant IsD consisted of tacrolimus, mycophenolate mofetil and high dose cyclophosphamide (days 3 and 4). He achieved complete chimerism on d + 28 and maintained one-year post transplant. He did not develop acute GVHD but experienced limited chronic GVHD (eyes and mouth) 14-18 weeks after HSCT for which he was started on low dose prednisone and topical steroids. One year after HSCT he received renal allograft from his father. He continued to take tacrolimus post-transplant, which was tapered off by nine months. Two years following his renal transplant, his serum creatinine is 1.19mg/dl and his MM remains in complete remission (Figure I).
Conclusion:
Stem-cell-based therapies used in order to induce durable transplant tolerance are currently under development. Data from early trials suggest successful and durable IsD tapering in patients with combined kidney and HSCT associated with prolonged graft survival. Further, comorbidities associated with allografts have been decreased including hypertension, diabetes, and hyperlipidemia. Data from other case centers also report successful and durable IsD tapering in patients with combined kidney and HSCT associated with prolonged graft survival. However, patients with combined kidney and HSCT did develop more AKI post-transplant. Other concerns related to combined transplant are sudden death, GVHD and secondary malignancy. Ongoing clinical trials are being conducted to induce transplant tolerance to achieve either mixed chimerism and full chimerism. Results from the early clinical trials look promising, but more data is needed to assess long-term safety and efficacy.
Siegel:Amgen: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Merck: Honoraria. Richter:Takeda: Consultancy, Speakers Bureau; Jannsen: Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau. Biran:Novartis: Speakers Bureau; Celgene: Speakers Bureau; Takeda: Speakers Bureau; Amgen: Speakers Bureau. Skarbnik:Abbvie: Consultancy; Seattle Genetics: Speakers Bureau; Gilead Sciences: Speakers Bureau; Genentech: Speakers Bureau; Pharmacyclics: Consultancy. Vesole:Celgene: Speakers Bureau; Novartis: Speakers Bureau; Takeda: Speakers Bureau; Janssen: Speakers Bureau; Amgen: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal