Abstract
Background: Allogeneic hematopoietic stem cell transplantation (HCT) is the only curative therapy for chronic lymphocytic leukemia (CLL). With this analysis we intended to identify prognostic factors in patients undergoing reduced-intensity conditioning (RIC) or non-myeloablative (NMA) HCT at our center in the immunochemotherapy era.
Methods: This retrospective chart review included all CLL patients who underwent RIC or NMA conditioned HCT at Memorial Sloan Kettering Cancer Center (MSKCC) in the immunochemotherapy era between 09/2006 and 10/2014. We then analyzed whether pre-HCT factors including age, disease response status at the time of HCT, presence of Richter's transformation, 17p deletion or TP53 mutation, use of anti-thymocyte globulin (ATG), number of prior lines of treatment, and time from diagnosis to HCT, were associated with overall survival (OS), progression-free survival (PFS), non-progression mortality, and grade 2-4 acute graft versus host disease (aGVHD). Univariate factors were evaluated via log-rank or Gray's test as appropriate, while Cox regression models were used to explore the adjusted effect of multiple factors.
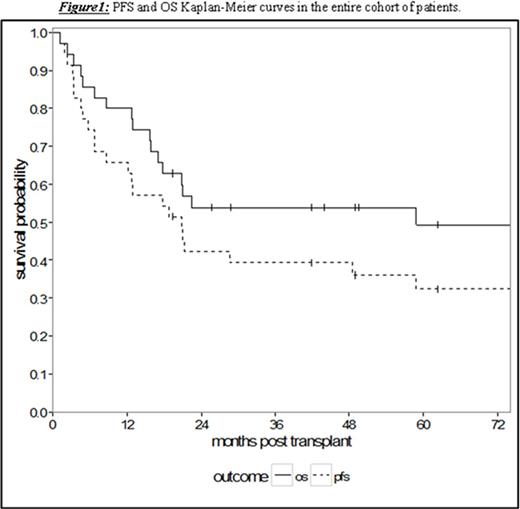
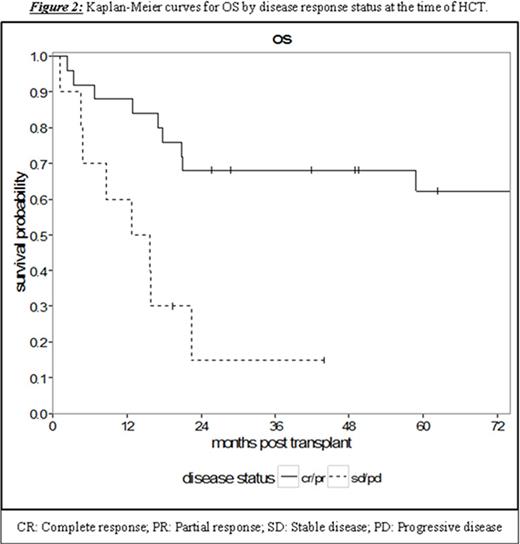
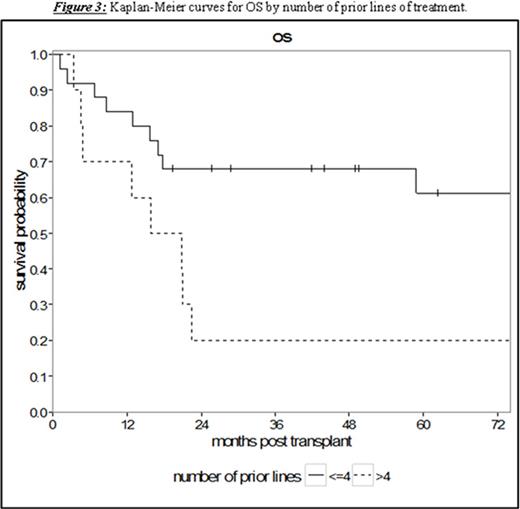
Results: Thirty-five patients undergoing RIC or NMA HCT with a median age of 53 years (range 36.3-69.0) were analyzed. The patients had a median of 4 prior lines of therapy (range 1-10) and a median time from diagnosis to HCT of 65.8 months (range 7.5-159.0). With a median follow-up for survivors of 58.8 months (95% CI 17.0-NA), the 5-year PFS and OS were 32.4% (95% CI 17.4-48.4) and 49.4% (95% CI 31.2-65.2), respectively (Figure 1). Treatment-related mortality was 20.0% (95% CI 8.7-34.7) and 31.8% (95% CI 17.0-47.6) at 1 and 2 years, respectively. Chemosensitive disease, defined by complete or partial remission per contemporary International Workshop CLL (iwCLL) response criteria at the time of HCT, was associated with improved 3-year OS of 68.0% (95% CI 46.1-82.5) compared to patients with chemorefractory disease to last line of therapy (stable or progressive disease) with a 3-year OS of 15.0% (95% CI: 1.0-45.7, p = 0.002, Figure 2). Additionally, patients with ≤4 lines of therapy prior to HCT experienced superior 5-year OS of 61.2% (95% CI 37.5-78.2) contrasted to 20.0% (95% CI: 3.1-47.5) in patients with >4 prior lines of therapy (p = 0.008, Figure 3). This difference approached statistical significance in multivariate analysis (HR 2.43, 95% CI 0.91-6.50; p = 0.076) adjusted for chemosensitivity. Furthermore, when adjusted for the number of prior lines of therapy, chemorefractory patients remained at greater risk of death in multivariate analysis (HR 3.29, 95% CI: 1.14-9.48, p = 0.027). Other factors including: age, history of transformed disease, the presence of 17p or TP53 deletion/mutation, time from diagnosis to HCT, or use of ATG with NMA/RIC did not impact PFS or OS.
Conclusion: This is the first study to report independent prognostic impact of the number of lines of therapy prior to NMA/RIC HCT on OS for CLL patients in the post-immunochemotherapy era. We confer findings from other groups including: the prognostic significance of chemosensitivity at the time of HCT as well as HCT overcoming poor-risk cytogenetics associated with 17p chromosomal aberrations. Our data is limited by lack of patients previously exposed to recently FDA approved novel kinase or BCL2 inhibitors. Nevertheless, given our reported data, HCT should still be considered as the only therapy with curative potential for poor-risk patients earlier in their disease course, while chemosensitivity is maintained and prior to the accumulation of multiple lines of therapies.
Perales:Incyte Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal