Abstract
Cryoglobulins (CGs) are immunoglobulins that precipitate at temperature below 37 C and re-dissolve after rewarming. Type I cryoglobulinemia is characterized by monoclonal immunoglobulins and is often driven by a lymphoproliferative disorder or plasma cell dyscrasia. Although CGs are often associated with hematologic malignancies which independently require therapy; this phenomenon can also be associated with more benign disorders. Monoclonal gammopathy of renal significance (MGRS) represents a group of disorders characterized by the presence of a paraprotein; although not fitting the diagnostic criteria for a symptomatic hematologic malignancy such as lymphoma or myeloma, can manifest by renal impairment through a variety of mechanisms. There exists no standard approach to manage these patients. As the driving force of these processes are characterized by the proliferation of malignant plasma cells (overproducing a nephrotoxic paraprotein) high-dose melphalan followed by autologous stem cell rescue represents an ideal approach to achieve organ function recovery and long term disease control
Our patient is a 56-year-old man who was initially evaluated for fatigue, lower extremity swelling and leg ulcers in December of 2011. Workup revealed worsening renal function and proteinuria. A renal biopsy was performed showing abundant cryofibrinogen deposits and cryoglobulinemic glomerulonephritis with membranoproliferative pattern. Labs showed type I cryoglobulinemia composed of monoclonal IgG kappa proteins. He was treated by his rheumatologist with a short course of prednisone and cyclophosphamide with transient improvement in proteinuria and renal function.
He was monitored without therapy until 2015 when the gradual worsening of his edema led him to be re-evaluated by a nephrologist who ultimately referred the patient for hematologic workup upon the discovery of a monoclonal protein. Bone marrow evaluation revealed 7% clonal plasma cells with FISH studies positive for gain of CKS1B, trisomy 9 and monosomy 13. Further evaluation did not confirm a diagnosis of symptomatic myeloma. At this time however, the creatinine had risen to over 2 with over 35 grams of proteinuria on a 24-hour urine specimen. Lab work confirmed the presence of a cryoglobulin.
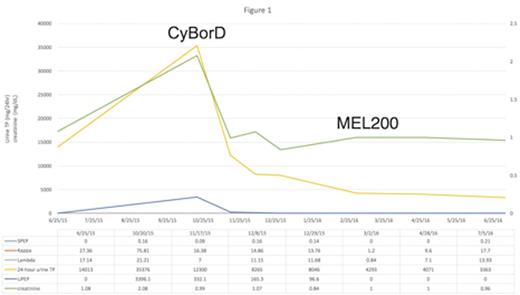
As the recurrent cryoglobulinemia was felt to be plasma cell driven and the etiology of the renal impairment; the decision was made to treat with Bortezomib, Cyclophosphamide and Dexamethasone (CyBorD). After 3 cycles of therapy the patient had normalization of his serum creatinine, marked improvement in edema, as well as significant improvement in proteinuria. Peripheral blood stem cells were mobilized following the administration of high-dose cyclophosphamide (2gm/m2) plus high dose filgrastim. He was subsequently conditioned for transplantation utilizing high-dose melphalan (HDM/200mg/m2) followed by autologous stem cell rescue. He tolerated the procedure well without any unexpected toxicities.
Evaluations post transplant revealed no evidence of a circulating cryoglobulin and continued clinical improvement and reduction in proteinuria. (figure 1). Now, 5 months post transplant, the patient has no evidence of a detectable paraprotein and has returned to his premorbid condition.
Type 1 Cryoglobulinemia associated renal disease represents a complicated disorder for which there is no standard of care. As this may be driven by clonal plasma cells; classical anti-myeloma therapy may provide an optimal strategy for management. Given the typical low plasma cell burden in these patients HDM has the potential to offer deep and durable remissions. Prospective studies are needed to validate this approach.
Biran:Amgen: Speakers Bureau; Takeda: Speakers Bureau; Celgene: Speakers Bureau; Novartis: Speakers Bureau. Skarbnik:Pharmacyclics: Consultancy; Gilead Sciences: Speakers Bureau; Genentech: Speakers Bureau; Seattle Genetics: Speakers Bureau; Abbvie: Consultancy. Siegel:Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Merck: Honoraria. Vesole:Amgen: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Speakers Bureau; Celgene: Speakers Bureau; Janssen: Speakers Bureau. Richter:Celgene: Consultancy, Speakers Bureau; Jannsen: Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal