Abstract
Background: High dose therapy (HDT) followed by autologous hematopoietic cell transplantation (autoHCT) has been shown to be safe and effective in patients with HIV-related lymphoma (HRL). Data is limited to small case series, transplant registries and a single prospective multicenter observational study. Here we report our institutional experience with auto-HCT in patients with HRL.
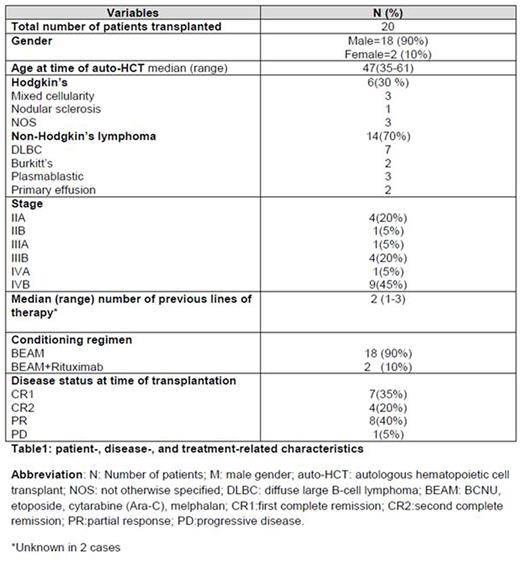
Patients and methods: Twenty patients with HRL [non-Hodgkin=14 (70%), Hodgkin=6 (30%)] and treatable HIV infection underwent HDT consisting of carmustine, etoposide, cytarabine and melphalan (BEAM) followed by peripheral blood auto-HCT from 04/2006 to 07/2015. In 2 cases rituximab was administered as part of the preparative regimen. Patient-, disease-, and transplant-related characteristics are summarized in Table 1.
Results: Median age was 48 years (range 35-61). The median follow-up for surviving patients was 42 months (range 6-110). At transplant, median peripheral blood CD4 count was 226 cells/µl (range 41-761). HIV viral load was undetectable in 14 out of 20 patients and lower than 4 logs in all of them. The median time to neutrophil and platelet engraftment were 11 days (range 10-13) and 14 days (range 13-176), respectively. Response rates at day +100 post-autografting in 17 evaluable patients were as follows: complete remission (CR)=11/17 (65%), partial response (PR)=2/17 (12%), and relapse/progression=4/17 (24%). Median event-free survival (EFS) was 58.4 months. Median overall survival (OS) was 74.3 months. At 5-years post-transplantation, EFS and OS were 68% and 53%, respectively. Non-hematologic toxicities consisted of mucositis in 8 (grade 1=3, grade 2=5), and enteritis in 13 patients (grade 1=2, grade 2=3, and grade 3=8). There were 13 documented infections in 11 patients (bacterial=9, viral=2, fungal=2). Six patients died from disease relapse/progression (n=5) and infection (n=1). Non-relapse mortality was 0% at day 100 and 5% at 5 years.
Conclusion: Patients with HRL and treatable HIV infection should be offered autoHCT if indicated. HIV infection is no longer a contraindication for autoHCT in this population.
Chavez:Janssen: Speakers Bureau. Kharfan-Dabaja:Seattle Genetics: Speakers Bureau; Incyte: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal