Abstract
Introduction
Chronic Graft Versus Host Disease (cGVHD) is a major complication of allogeneic stem-cell transplantation and is characterized by frequent multi-organ involvement that resembles the autoimmune diseases. Donor-derived CD4+ and CD8+ T lymphocytes have classically been considered to be the main effector cells mediating GVHD pathogenesis. Indeed, removal of T cells from transplant inocula almost completely prevents GVHD developing, at the price of increased incidences of graft rejection and disease recurrence. However recent studies suggest that B cells might also play an important role in the biology of cGVHD. The role of Treg lymphocytes in the pathogenesis of cGVHD is still controversial and the tyrosine kinase inhibitor′s (TKI) role in the modulation of this pathway is not yet fully characterized. In vitro data confirm that TKIs regulates both innate and adaptive immune response by interacting with many cell population such as T-cells, B-cells, dendritic cells, mast cells and macrophages.
According to these observations, we investigated the TKI′s immunomodulatory effects (Nilotinib, Dasatinib, Imatinib, Ponatinib) on lymphocyte populations.
Materials and Methods
Peripheral blood mononuclear cells were isolated by density gradient centrifugation using Ficoll-Biocoll. Cells were cultured in RPMI 1640 at a concentration 1x106 cell/well. Nilotinib, Imatinib, Dasatinib and Ponatinib were added to cell cultures at serial concentration (Imatinib:1μM,10μM,50μM; Nilotinib:0.5μM,2μM,10μM; Dasatinib:50nM,100nM,200nM; Ponatinib:1nM,10nM,50nM,100nM) on the first day.
Six-color flow cytometry analysis (Facs Canto II) was performed on the cells harvested after 96 h cultures using conjugated antibodies (CD3,CD4,CD16,CD56,CD3,CD25,CD19,CD45RA,FoxP3,CD127,7-Aminoactinomycin-D), for cell cycle analysis cells were stained with propidium iodide.
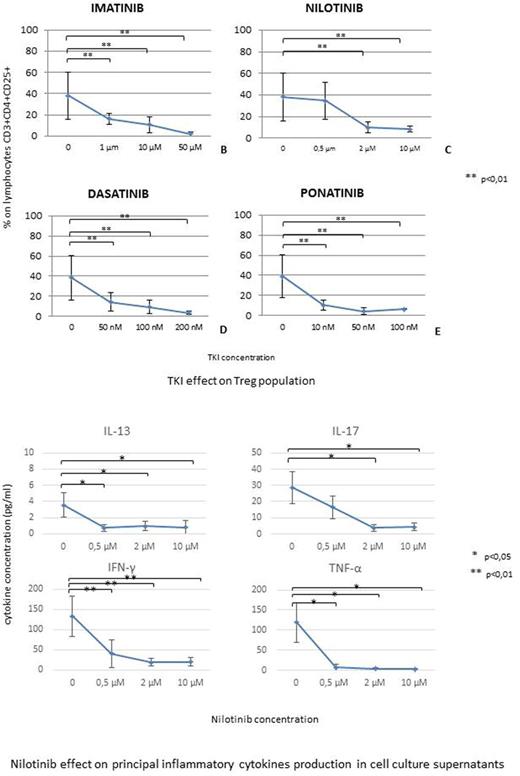
For cytokine analysis, supernatants were collected and analyzed for cytokines according to the instruction of Bio-Plex Pro Human Cytokine 17-plex Assay with Bio-Plex (Bio-Rad).
Results
A significant decrease of cytotoxic T cells viability was observed when cells were cultured in presence of Imatinib (50μM,p<0.01), Ponatinib (10nM,p<0.05) and Dasatinib (100nM,p<0.01). On the contrary, exposure to Nilotinib didn′t induce cell death. Increasing concentrations of all the tested TKI significantly inhibited T cell proliferation in a dose-dependent manner; the effect become statistically significant starting from Imatinib (1μM,p<0.05), Dasatinib (50nM,p<0.01), Ponatinib (50nM,p<0.01) and Nilotinib (0.5μM,p<0.01).
Exposure to Imatinib, Dasatinib and Ponatinib induced a statistically significant decrease (p<0.01) of Treg cells proportion, even at the lowest drug concentration in culture; Nilotinib induced Treg decrease only at concentrations exceeding 2μM (p<0.01), higher than those usually achieved in clinical practice.
A significant increase of naive Treg apoptosis was observed after exposure to Dasatinib (50nmM,p<0.01), Ponatinib (50nM,p<0.01) and Imatinib (50μM,p<0.01); exposure to Nilotinib has no effect on this population.
Both Nilotinib and Dasatinib induced a profound inhibition of pro-inflammatory cytokine production (in particular TNFα, IFNγ, IL13 and IL17) when added to the cell cultures (p<0.05); slower decrease in supernatant cytokine concentration was observed in presence of either Imatinib (50μM,p<0.05) and Ponatinib (50nM,p<0.05).
Increasing concentrations of all TKIs except Nilotinib induced a significant decline of NK cells (p<0.01) and B cell (p<0.01).
Conclusion
The present study focuses the peculiar Nilotinib activity on lymphocyte′s regulation: this TKI, at therapeutic concentrations in vitro, interact with innate and adaptive immune response show anti-inflammatory properties. Unlike other TKIs, Nilotinib determine inflammatory cytokines reduction, preserving T cell population and Treg. These data support the potential use of Nilotinib in cGVHD
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal