Abstract
Background & Aim: Recombinant human soluble thrombomoduline alpha (rhTM) is a novel anticoagulant agents and approved for disseminated intravascular coagulation (DIC) in Japan. Although several case reports suggested its therapeutic potential for sinusoidal obstructive syndrome/hepatic veno-occlusive disease (SOS/VOD), no information is available in large cohort. The aim of the study is to evaluate the therapeutic potential of rhTM for SOS/VOD.
Patients & Methods: We retrospectively studied 878 times of allogeneic hematopoietic cell transplantation (HCT) in Toranomon Hospital from June 2008 to June 2015. We extracted the patients who used rhTM for DIC and satisfied the diagnostic criteria of SOS/VOD around the same time, because the use of rhTM for SOS/VOD alone is off-label. We excluded the patients who were already treated with rhTM before the emergence of the first symptom or sign of SOS/VOD, who used rhTM only in the short period (within 2 days), and who started rhTM over 30 days after the emergence of the first symptom or sign of SOS/VOD. To diagnose classical SOS/VOD (≤ 21 days after transplantation), we used two classical criteria of the modified Seattle (McDonald et al. Ann Intern Med 1993) and the Baltimore (Jones et al. Transplant 1987). For late-onset SOS/VOD (> day 22 of transplantation), we used the criteria which was recently published from the EBMT group (Mohty et al, Bone Marrow Transplant 2016). We considered the atrophic change of the liver in a long-term clinical course as an evidence of SOS/VOD, in a patient who was not evaluated the portal flow. We defined as severe SOS/VOD, if the patients had renal (Cr ≥ 2 times of baseline at transplant), respiratory (SpO2 ≤ 90% and/or the need for positive pressure) or central nervous system failure (confusion, lethargy and/or delirium) until 2 weeks after the diagnosis of SOS/VOD. Complete response (CR) of SOS/VOD was defined as the resolution of all the symptoms and the signs of SOS/VOD diagnostic criteria. We prioritized the date of Baltimore criteria, when we fixed the date of diagnosis.
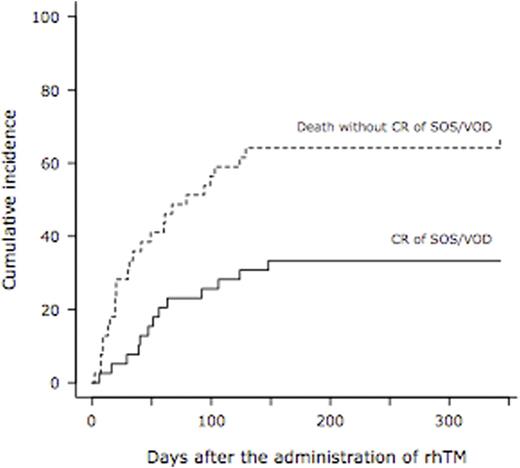
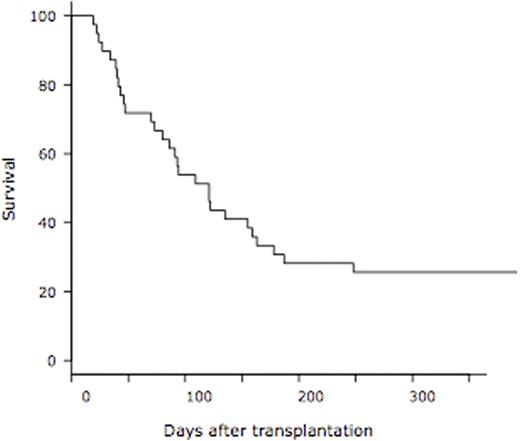
Results: A total of 39 patients used rhTM for DIC and concurrent SOS/VOD. The median age was 60 years (range, 27 - 72) and 27 patients (69%) was male. Hematologic diseases were as follows; AML (n=25), ALL (n=5), lymphoma (n=3), CML (n=2), MDS (n=2) and CMML (n=2). Donor cell sources were UCB (n=34) and UBM (n=5). Most of the prophylaxis regimen at the time of transplantation was the combination of ursodeoxycholic acid and dalteparin in 36 patients (92%). Classical SOS/VOD was diagnosed in 3 (8%) and 8 patients (21%) by the criteria of the modified Seattle and the Baltimore at the median day of 14 (range, 11 - 14) and 16 (range, 11 - 20), respectively. Twenty-eight patients (72%) were diagnosed as late-onset SOS/VOD at the median day of 44 (range, 22 - 89). In a total of 39 patients, severe SOS/VOD developed in 33 patients (85%) (renal, n=32; respiratory, n=7; central nervous system, n=15). The elevation of transaminase (2x upper limit of normal range) was observed in 18 patients (46%). The median interval from the emergence of the first symptom or sign of SOS/VOD to rhTM administration was 7 days (range, 0 - 23). The median duration of rhTM administration was 11 days (range, 3 - 63). RTM was used alone in 20 patients (51%), in combination with dalteparin in 7 (18%), with antithrombin III (ATIII) in 5 (13%), with dalteparin & ATIII in 3 (8%), with ATIII & prostaglandin E1 (PGE1) in 2 (5%), and with PGE1 in 2 (5%). Corticosteroid was used with rhTM concomitantly in 32 patients (82%). Finally, 13 patients achieved CR of SOS/VOD. The cumulative incidence of CR of SOS/VOD was 33.3 % at 1 year after the administration of rhTM (95% confidence interval, 18.5 - 48.9%) (Figure 1). The median interval from the administration of rhTM to CR of SOS/VOD was 51 days (range, 6 - 141). At 1 year after transplantation, overall survival was 25.6% (95% confidence interval, 13.3 - 69.9%) (Figure 2). From the administration of rhTM to 2 weeks after the cessation of rhTM, 23 hemorrhagic adverse events were observed. Seven out of 23 events were at grade 3-5, and 5 out of 7 events were fatal (intra-abdominal n=2, gastro-intestinal n=1, lung n=1, and brain n=1).
Conclusion: We concluded that rhTM had a possible therapeutic potential for SOS/VOD. Its safety and efficacy should be evaluated in a prospective study in the future.
Izutsu:Abbvie: Research Funding; Gilead: Research Funding; Celgene: Research Funding; Janssen Pharmaceutical K.K.: Honoraria; Eisai: Honoraria; Kyowa Hakko Kirin: Honoraria; Chugai Pharmaceutical: Honoraria, Research Funding; Takeda Pharmaceutical: Honoraria; Mundipharma KK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal