Abstract
Background
Allogeneic hematopoietic stem cell transplantation (HSCT) has been used to treat some of hematological malignancies and inherited or acquired non-malignant diseases. Unfortunately, graft-versus-host disease (GVDH) occurred approximately 15% in transplant recipients and decreases the success of allogeneic HSCT. Currently, isolation of natural regulatory T cells (nTregs) and in vitro-expanded nTregs was shown to be an effective therapy to GVHD patients. However, shortage of nTregs in peripheral blood and time consumption of expansion in vitro may eventually limit the clinical application. Conversely, induction regulatory T cells (iTregs) can be generated in vitro from naïve T cells and to a large number of iTregs in short time. It may improve the GVHD therapeutic efficiency. The stability of FoxP3 in iTregs is a crucial factor for suppression of GVHD. Besides, a functional iTreg cell should be CD127 negative. Because of that, we should provide a large number of effective iTreg cells, the CD4+CD25+FoxP3+CD127-iTregs, to ensure the successful therapy.
Aim
In order to harvest the large number of effective iTregs for preventing the GVHD, we try to develop a method for induction of more iTreg cells via expansion and repeated activation of naïve T cells.
Methods
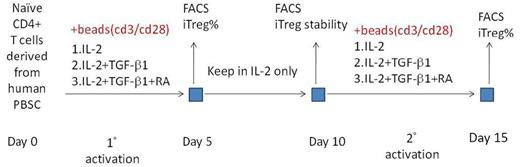
Human PBSC were prepared from peripheral blood of health donor by Ficoll-Hypaque density gradient centrifugation. Naïve T cells were isolated by negative selection. The harvested naïve T cells were cultured and stimulated under cytokines-containing RPMI1640 medium. The flow chart was shown in figure. For cell proliferation, the cells were cultured under IL-2 supplemented medium. The harvested cells were analyzed by flow cytometry with fluorescence-conjugated CD antibodies, including CD4, CD25, CD127 and FoxP3.
Results
With our purpose, harvest more iTreg cells should be efficient for clinical application. Amplification of the T cell number can obtain more iTreg cells; therefore, cultivation of the T cells under IL-2-containing medium would stimulate the cell proliferation to about 4-fold on the 10th day. After the first cytokines stimulation, we harvested the iTregs, and then, keep the cells in IL-2 only medium for another 5 days to amplify the T cells. On the 10th day, naïve T cell reactivation would be performed with anti-CD3/CD28 antibodies and with cytokines supplement for the second induction. The number of iTregs would increase to about 20-40%. It indicated that we would harvest more iTreg cells for application. If we cultured the naïve T cells under IL-2 medium in the beginning, we would not obtain much more iTregs, comparing with the reactivation method.
Conclusion
Our study showed that after the first activation and induction of naïve T cells, we could expand more cells under IL-2 containing medium. Then, after the second activation and induction, we could harvest more iTreg cells markedly. The result should develop a novel-cell based approach for potentially reducing the risk of GVHD within short time.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal