Abstract
Aim: To compare patients with newly diagnosed multiple myeloma (NDMM) withbortezomib responsive and resistant disease (defined as <PR after 2 cycles) and assess the impact on clinical outcome particularly progression-free survival (PFS) and overall survival (OS).
Method: Retrospective review of all patients identified with NDMM treated with upfront bortezomib in four tertiary centres in Victoria, Australia between 1st of January 2009 and 1st of March, 2016. Bortezomib responsiveness was defined as achievement of PR or better after completing 2 cycles of any bortezomib containing therapy (total eight doses of bortezomib). Prior radiotherapy was allowed. Data were analysed via log rank tests for PFS and OS using SAS (V9.4) software. Predictors of bortezomib resistance were assessed by chi-square analysis.
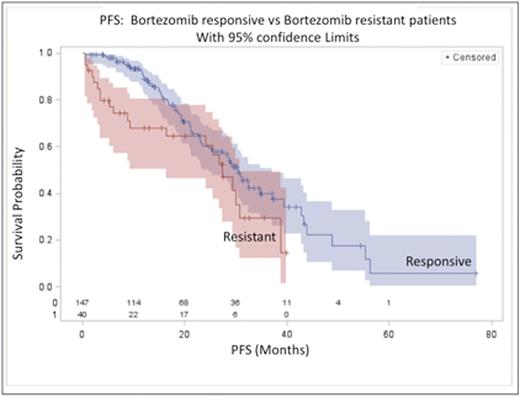
Result: A total of 187 eligible patients were included (147bortezomib responsive and 40bortezomib resistant patients). Bortezomib resistance was associated with shorter median PFS, 27.4 (95%CI: 16.3, 30.8) vs 30.4 (95%CI: 23.4, 37.1) months (log-rank p= 0.047) with a median potential follow up of 27.4 vs 29.7 months in the resistant and responsive groups respectively. Estimated PFS at 12 months was 68.0% (95%CI: 50.3%, 80.5%) vs 88.3% (95%CI: 81.2%, 92.8%) and, at 24 months, 60.4% (95%CI: 41.7%, 74.7%) vs 60.0% (95%CI: 49.9%, 68.6%) in the resistant and responsive groups respectively, indicating non-constant hazard in the resistant group (see figure). Bortezomib resistance was not associated with higher ISS (ISS3 24% vs 15%, p=0.17), high riskcytogenetics (17p del ort(4;14)) (23% vs 23%, p= 0.99), MM sub-type (p= 0.63) or length ofbortezomib cycle (21 vs 35 day p= 0.56)). In responders (n=147), maintenance therapy was associated with improved PFS (p=0.021), and PFS at 24 months was, 70.7% (95%CI: 57.3%, 80.5%) vs 46.9% (32.0%, 60.5%) in those who received maintenance therapy and those who did not respectively. In non-responders (n=40) PFS at 24 months was 68.8% (95%CI: 35.7%, 87.3%) vs 54.9% (31.6%, 73.2%) in those patients who received maintenance therapy and those patients who did not respectively (p=0.84). Median OS has not been reached in either thebortezomib resistant or responsive groups however estimated OS at 24 months is 82.8% (95%CI: 62.8%, 92.7%) vs 89.5% (95%CI: 81.5%, 94.1%) in the resistant and responsive groups respectively.
Conclusion: Bortezomib resistance is a poor prognostic factor and is associated with a shorter PFS. Consideration of early therapy change, maintenance therapy and/or referral for assessment for allograft in eligible patients appears to be warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal