Abstract
Introduction
For chronic myelomonocytic leukemia (CMML) several scores exist which prognosticate overall survival (OS) based on different clinical and genetic parameters. The time-to-treatment (TTT) among CMML patients is highly variable, and a predictive model to specifically estimate TTT in CMML has not been described so far. The aims of this single-center retrospective study were (a) to test and validate established myelodysplastic syndrome (MDS)-specific and CMML-specific prognostic scores in our patient cohort, (b) to evaluate which baseline factors were relevant to the time point of treatment initiation with either hydroxyurea or azacitidine, and (c) to propose a prediction model for TTT in CMML.
Methods
This retrospective analysis was based on the data of 55 unselected, consecutive CMML patients diagnosed and/or treated at our tertiary center between 2004 and 2015. We applied the following published prognostic models to our CMML cohort, using both OS and TTT as endpoints: the MD Anderson Prognostic Score (MDAPS), the modified MDAPS (MDAPS M1), the CMML-specific Prognostic Scoring System (CPSS), the Mayo Prognostic Model, the Düsseldorf Score, the International Prognostic Scoring System (IPSS), and the Revised International Prognostic Scoring System (IPSS-R).
Results
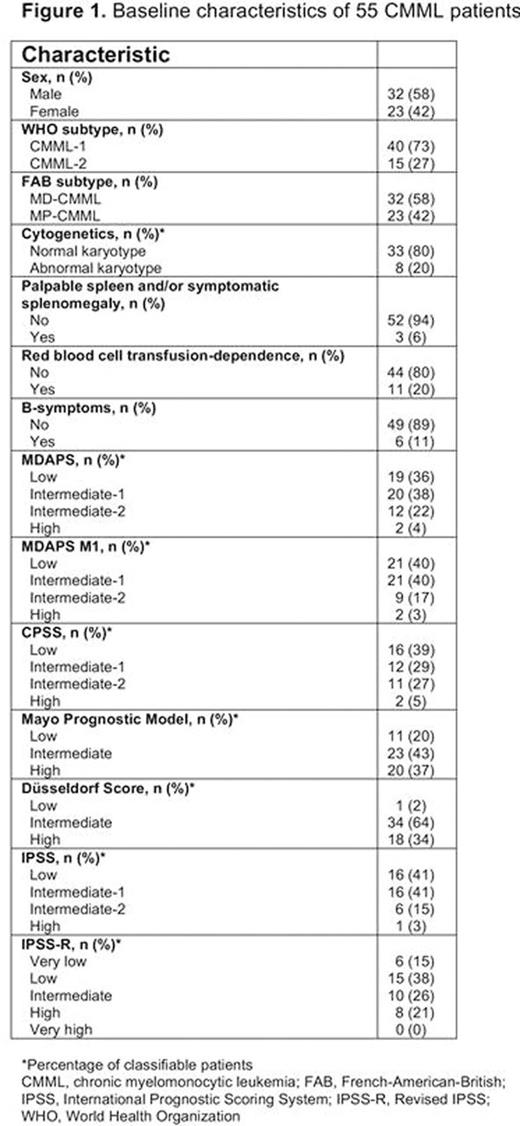
According to the CMML-specific MDAPS, 27% of our patients were classified as "higher-risk" (23% intermediate-2, 4% high-risk) (Figure 1). At the time of data analysis, 38% and 24% of patients had received azacitidine and hydroxyurea as first-line treatment. A total of 40 (73%) patients had died at the time point of data analysis. The median time of follow-up was 24.8 months (range 1.7-74.8 months).
All applied MDS-specific (Düsseldorf Score, IPSS, IPSS-R) and CMML-specific (MDAPS, MDAPS M1, CPSS, Mayo Prognostic Model) prediction scores were able to significantly discriminate patient cohorts with different OS probabilities. The following variables were associated with a shorter TTT in the univariate analysis: the presence of immature myeloid cells in the peripheral blood, white blood cell count ≥14.5 G/L, platelet count <55 G/L, absolute neutrophil count ≥6 G/L, absolute lymphocyte count ≥2.3 G/L, absolute monocyte count ≥2.8 G/L, serum lactate dehydrogenase ≥223 G/L, peripheral blood blasts >0%, bone marrow blast percentage ≥7.5%, red blood cell transfusion-dependence, palpable spleen and/or symptomatic splenomegaly, and the presence of B-symptoms at the time of initial diagnosis. In multivariate analysis, the following factors remained independently associated with TTT: lactate dehydrogenase (HR 5.428; p = 0.008), bone marrow blast count (HR 4.570; p = 0.001), and platelet count (HR 2.660; p = 0.027).
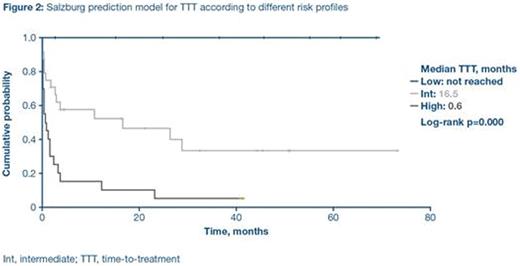
These three clinical parameters were included in the TTT prediction model and CMML patients were stratified into three subgroups: low-risk, intermediate-risk and high-risk. Median TTT was not reached for low-risk patients, 16.5 months for intermediate-risk patients, and almost immediate treatment initiation (0.6 months) was observed in the high-risk group (Figure 2).
Conclusions
We validated seven existing MDS-specific and CMML-specific prognostic scores in 55 CMML patients treated at the center in Salzburg. We were able to demonstrate that lactate dehydrogenase, bone marrow blast percentage and platelet count at initial diagnosis were the most relevant parameters for predicting time to treatment initiation in our CMML cohort. Based on these three parameters, we propose the first TTT prediction score for treatment-naïve CMML patients. Clinical implications of this score include the identification of CMML patients for early investigational trials, as well as the tailoring of individual follow-up intervals.
Huemer:Roche: Other: Travel funding; Merck: Other: Travel funding. Egle:Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: travel support; Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: travel support. Greil:Pfizer: Honoraria, Research Funding; Boehringer-Ingelheim: Honoraria; Eisai: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Mundipharma: Honoraria, Research Funding; Merck: Honoraria; Janssen-Cilag: Honoraria; Genentech: Honoraria, Research Funding; Novartis: Honoraria; AstraZeneca: Honoraria; Roche: Honoraria, Research Funding; Sanofi Aventis: Honoraria; GSK: Research Funding; Ratiopharm: Research Funding; Cephalon: Consultancy, Honoraria, Research Funding; Bristol-Myers-Squibb: Consultancy, Honoraria. Pleyer:Celgene: Consultancy, Honoraria; Bristol-Myers-Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; AOP Orphan Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal