Abstract
Background. L-asparaginase (L-ASPA) based-chemotherapy (chemo) is one of the most efficient treatment for patients with extra-nodal NK/T nasal type lymphoma, in front- line or at relapse. This treatment is combined either with radiation therapy (RT) if localized or with high dose therapy plus autologous stem cell transplantation (HDT/ASCT) if disseminated. However it may be associated with toxicities that may impair efficacy and survival.
Aim. The aim of this study was to report the real-life experience of L-asparaginase use in patients with NK/T nasal type lymphoma treated with L-ASPA based-chemotherapy and to identify risk factors associated with 1/ main adverse events and 2/ survivals.
Patients and methods. Between July 2003 and June 2016, 28 patients were treated in our center for NK/T nasal type lymphoma. Four of them were excluded because they did not receive L-ASPA- based chemotherapy. Among the 24 pts included, 21 received the L-ASPA based-chemo as front-line therapy, and 3 pts as second line, either in association with gemzar and dexamethasone (n=14), or with methotrexate and dexamethasone (n=6), or with etoposide and dexamethasone (n=1). RT was delivered in 18 pts; HDT/ASCT in 3 pts; and both in 1 pt. Median number of injections of L-ASPA was 12 per patient (range 3-32). Grade 3-4 of toxicities (tox) were retrospectively assessed according to CTCAE V4.03, with a particular attention to 4 categories: hematological, hemostasis, liver and pancreatic dysfunctions. Progression-free (PFS) and overall survivals (OS) were analyzed from time of first L-ASPA injection, based on Kaplan Meier estimates.
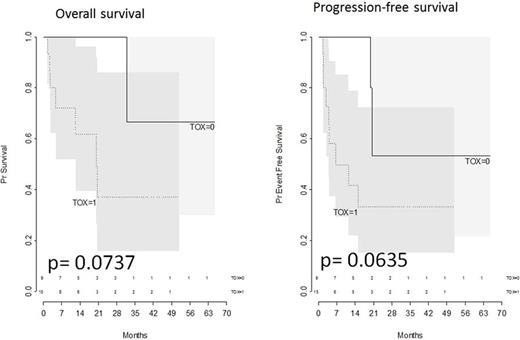
Results. Median age at diagnosis was 51 years (interquartile range, IQR : 40.7-61, ranging from 24-78). Sex ratio M/F was 0.71. PS was > 2 in 3 patients. Median body mass index (BMI) was 24 kg/m 2 (IQR: 23-25.7, ranging from 17-33). Stage was localized in nasal site in 20 patients, and disseminated (III-IV) in 4 patients, with bone marrow involvement in 2 pts, muscle in 1 pt, skin in 1 pt, and extensive nasal in 1 pt. LDH level was high in 10 (41.7%) patients. At diagnosis, EBV serum DNA was positive in 15/20 (75%) patients. All patients were negative for HBV, HIV, HCV. Abnormal baseline levels of ASAT/ ALAT (> 2N) were present in 2 patients, amylase/lipase (>1.5N) in 2 pts, bilirubine (2N) in 4 pts. Baseline anemia (< 10g/L) and thrombopenia (< 100 G/L) were present in 6 and 2 patients, respectively. Hemostastic parameters were normal in all patients. During the treatment, 10 (42%) patients developed gr3-4 hepatic toxicities, and 3 (13%) pts pancreatic toxicities, 6 (25%) pts hematologic toxicities, 3 (13%) pts hemostatic toxicities. These toxicities induced a reduction of treatment in 6 (25%) patients. Hematological toxicities were associated to baseline hemoglobin and platelets levels and to poor PS (p= 0.008; p= 0.014 ; p= 0.095, respectively). Hemostatic toxicities were associated to the stage of the disease, but not significantly (p= 0.076). No factor was associated for hepatic or pancreatic toxicities. Age and BMI were not significantly associated to any toxicity. A complete remission was achieved at the end of treatment in 12 patients (50%). At 2 years, progression- free survival and overall survival were 39% (95%CI, 20.3-76%) and 51.5% (95%CI, 30-89%), respectively. Presence of toxicities impacted the progression free survival and overall survival, though not significantly (p= 0.0635 ; p= 0.0737) (Figure 1)
Conclusion. In this small retrospective analysis, we observed an impact of toxicities related to L-ASPA on the PFS and the OS of patients with extra-nodal NK/T lymphoma. The identification of the risk factors associated to these toxicities needs to analyze large cohorts of prospective trials.
Brice:Takeda Pharmaceuticals International Co.: Honoraria, Research Funding; Seattle Genetics: Research Funding; Roche: Honoraria; Gilead: Honoraria; Bristol Myers-Squibb: Honoraria. Thieblemont:Roche: Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal