Abstract
Background:
Follicular lymphoma (FL) is the second most common lymphoma in adults. Although responsive to various therapies it is considered incurable. The CD20 antibody rituximab is well known to have improved outcome, acting through complement-mediated cytotoxicity, antibody-dependent cellular cytotoxicity (ADCC) and direct induction of apoptosis. To enhance the efficacy of rituximab different combination regimens have been used, mostly with chemotherapy but also with cytokines and immunomodulatory agents such as lenalidomide.
Lenalidomide has been shown to induce durable responses with manageable toxicity in indolent lymphomas and mantle cell lymphoma. It modulates signaling pathways, enhances the capacity of T cells and natural killer (NK) cells and suppresses angiogenesis. When combined with rituximab, effects seem to be synergistic (Fowler 2014).
Aim:
To investigate the dynamics of immune cells in blood in patients treated with rituximab with or without lenalidomide.
Patients and Methods:
FL patients included in a multicenter randomized phase II trial performed by the Swiss Group for Clinical Cancer Research (SAKK) in collaboration with the Nordic Lymphoma Group (NLG) were randomized 1:1 to treatment either with rituximab alone or rituximab and lenalidomide. Inclusion criteria were histologically confirmed CD20+ FL grade 1, 2 or 3A, disease stage Ann Arbor III-IV (or II not suitable for radiotherapy) and need of therapy. In both treatment arms rituximab was administered as 4 single infusions of 375 mg/m2 weeks 1, 2, 3 and 4; patients who showed at least a minor response received 4 additional infusions at weeks 12, 13, 14 and 15. In the combination arm lenalidomide 15 mg p.o. daily was started 14 days before the first infusion and given continuously until 14 days after the last.
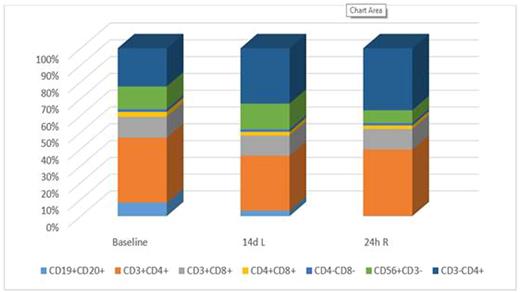
Blood cells were sequentially sampled: at baseline, after 2 weeks' of lenalidomide (combination arm solely), 24 hours after the first rituximab infusion and at follow-up weeks 10 and 23. Analyses of CD4+, CD8+, CD56+CD3- (NK) cells, CD19+CD20+ and CD3-CD4+ (mainly monocytes) were performed with flow cytometry and measured as percentages of mononuclear cells. T cell subpopulations CD27+CD45RA+ (naïve), CD27+CD45RO+ (memory) and CD26-CD45RA+ (effector) were measured as percentages of CD3+CD4+ and CD3+CD8+, respectively.
Results:
Rituximab and lenalidomide: Immune cell levels were analyzed in 13 Norwegian and Swedish patients randomized to combination treatment. Compared to baseline, the fraction of CD3-CD4+ cells increased in all but two (one of whom had an additional population of CLL cells), accompanied by decrease in CD4+ cells. As previously described NK cell levels rose (ASH 2015 abstract 1535), here in 9/12 patients. In 8/12 the CD4/CD8 ratio decreased.
Among CD4+ effector cells were few at baseline. Among CD8+ they were more abundant but decreased with lenalidomide. In both CD4+ and CD8+, naïve cells were reduced in favor of memory cells.
7 patients out of these 13 had blood samples retrieved 24h after first rituximab infusion. The B cell depletion initiated already with lenalidomide, was deepened. The NK cell fraction increased, as did fractions of naïve T cells, for CD4+ in 7/7 patients and for CD8+ in 6/7.
Figure 1: Average changes in immune cell composition among 7 patients treated with lenalidomide and subsequent rituximab.
Single rituximab: Among 7 rituximab treated patients sampled both at baseline and 24h post rituximab, NK cells decreased markedly in all but one. B cells were reduced as predicted. In comparison with 7 patients treated with rituximab and lenalidomide, the combination arm showed a relatively larger fraction of memory T cells.
Conclusion:
Changes in immune cell subsets were found in peripheral blood of FL patients after rituximab with or without lenalidomide. After lenalidomide alone, T cells developed from naïve to memory stages, and an increase in monocyte and NK cell fractions was found, the latter decreasing again after first rituximab as previously seen. Between treatment arms differences in memory T-cells was seen also after the first rituximab dose. Clinical outcome in relation to patterns of immune cell response is under analysis.
Wahlin:Roche: Consultancy. Kimby:Gilead: Honoraria; Baxalta: Consultancy; Abbvie: Consultancy, Honoraria; Jansen: Consultancy, Honoraria; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal