Abstract
Introduction
Follicular lymphoma (FL) is the most common form of indolent non-Hodgkin lymphoma in the United States. Although one disease, the histology, prognosis, and response to therapy for FL is quite heterogeneous. Recent large prospective studies including the BRIGHT study (Flinn et al.) and another by Rummell et al. have established Bendamustine-Rituximab (BR) as the optimal frontline regimen in pts with Grade 1-2 FL. However, the best management of Grade 3A FL remains a controversy, as pts with this diagnosis were excluded from these two important studies. Many clinicians have extrapolated data from these large prospective trials and are administering frontline BR chemotherapy for Grade 3A, while others approach it as an aggressive lymphoma and treat with anthracycline based regimens. In this study, we evaluate clinical outcomes of pts with Grade 3A FL at diagnosis by frontline therapy.
Methods
We performed a retrospective, single center analysis of all adult patients (pts) with a new diagnosis of Grade 3A FL since 2004. Pts with Grade 1-2, Grade 3B, and/or concurrent transformed lymphoma were excluded from analysis. Descriptive statistics were used to measure baseline characteristics. Comparative analysis was done using Fischer's exact test for categorical variables and ANOVA for continuous variables. Our primary endpoint was time to progression (TTP) after frontline therapy, which was estimated from the date of first treatment (Tx) to the date of progression or last follow-up. Secondary outcomes included overall survival (OS) and rates of transformation to aggressive lymphoma. Frontline Tx was categorized into 4 groups - anthracycline, bendamustine, rituximab alone, and other regimens. Stage at diagnosis was stratified as either 1-2 versus 3-4 and FLIPI as 0-2 versus 3-5.
Results
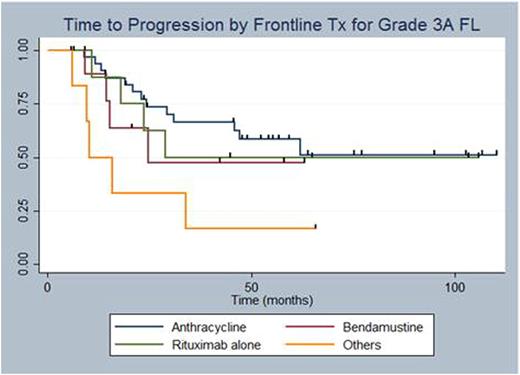
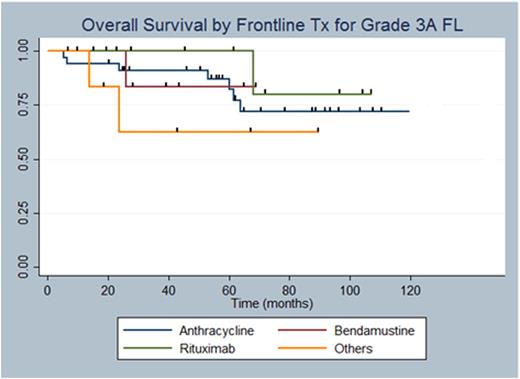
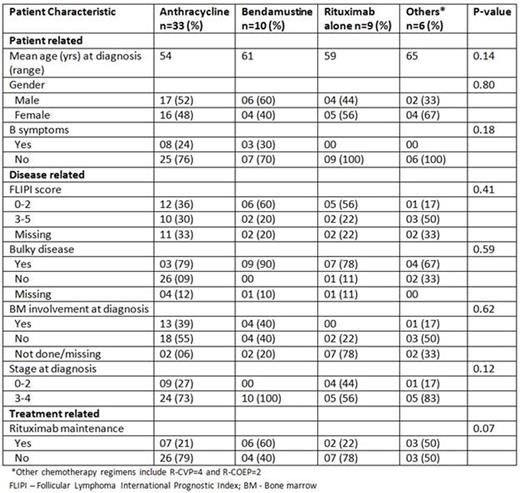
A total of 80 pts with Grade 3A FL were identified using the clinical and pathology databases. There was insufficient follow-up information on 22 pts and hence only 58 were analyzed for clinical outcomes including TTP, OS, and large cell transformation. Among the four groups, there was no difference in mean age, sex, stage at diagnosis, presence of "B" symptoms, bulky disease, or BM involvement (Table 1). We retrospectively calculated FLIPI for all pts with evaluable data (N=41) and there was no difference between the two groups (p=0.41). 57% of pts received anthracycline based chemotherapy while only a minority received bendamustine chemotherapy (17%). It is interesting to note that 21% (7/33) of pts treated with anthracycline chemotherapy received maintenance rituximab, while 60% (6/10) of pts who were treated with bendamustine upfront got maintenance rituximab (p=0.07). This is consistent with practice variation over time as bendamustine is a more contemporary agent. 12% (N=7) of the pts with Grade 3A FL developed in their course large cell transformation. The Kaplan-Meier (KM) curve in Figure 1 shows TTP and OS by the four Tx groups. There was a trend towards statistical significance favoring anthracycline therapy (log rank test, p=0.07), but these results are limited by difference in median follow-up between the four groups. With regards to OS, there was no statistically significant difference between the regimens (log rank test, p=0.58).
Conclusions
The optimal management of Grade 3A FL remains unclear. Whether it should be managed as an aggressive disease with anthracycline-like regimens or as an indolent lymphoma with bendamustine is an unanswered question. Histologically, Grade 3A FL has increased number of centrocytes and generally higher proliferation rates, which theoretically makes it more chemosensitive and perhaps more responsive to higher intensity regimens. Our study demonstrates a signal favoring anthracycline over other Tx modalities in prolonging TTP, but is limited by the low number of pts who received bendamustine based treatment, as it has only recently become a favored frontline therapy. OS was not different as expected due to multiple second line regimens available for relapsed pts. Future studies are indicated to determine the best frontline option for Grade 3A FL.
Fenske:Celgene: Honoraria; Millennium/Takeda: Research Funding; Pharmacyclics: Honoraria; Seatle Genetics: Honoraria. Hamadani:Janssen: Consultancy; Celgene: Honoraria, Research Funding; Takeda Pharmaceuticals: Research Funding. Shah:Oncosec: Equity Ownership; Exelixis: Equity Ownership; Geron: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal