Abstract
BACKGROUND: Acute erythroid leukemia (AEL) is categorized as an M6 acute myeloid leukemia (AML) by the French American British (FAB) classification system and now as either pure erythroleukemia or as a myelodysplastic syndrome (MDS) in the World Health Organization (WHO) 2016 classification. Traditionally, patients with AEL have a poor outcome; however, we have observed favorable responses to decitabine in these patients at our institution. Thus, we conducted a retrospective exploratory study to characterize our experience with the use of decitabine compared with other treatment approaches in patients with the diagnosis of AEL. We also characterize how these cases would now be classified according to the 2016 revisions to the WHO classification of myeloid neoplasms and acute leukemia.
METHODS: Adult patients diagnosed with AEL at the University of Michigan Health System from 2001-2015 were included. Patients were identified through the institutional hematopathology clinical software system and data were extracted from the electronic medical record. Treatment response was defined by the International Working Group and European Leukaemia Net Response definitions for AML. Standard definitions for overall survival (OS), event free survival (EFS), and progression free survival (PFS) were used.
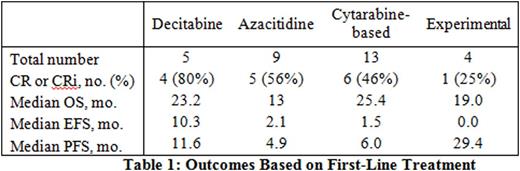
RESULTS: Thirty-six patients were identified; 11 were women and 25 were men. Median age was 68 years. Median OS of the entire cohort was 13.2 months. Patients treated with decitabine as first-line therapy had an 80% CR/CRi, a 23.2-month median OS, and a non-significant trend toward longer EFS and PFS compared to other treatments (Table 1). OS was numerically higher in those receiving decitabine compared with azacitidine, although not statistically significant, likely due to small patient numbers (Table 1). When compared to cytarabine-based induction regimens, decitabine regimens showed comparable OS and a non-significant trend toward longer EFS and PFS (Table 1). 4 of 5 patients received decitabine according to Blum, et al.1 (20 mg/m2 x 10 days). Five patients received best supportive care only. All 5 were diagnosed between 2005 and 2010, prior to the publication of decitabine use in elderly AML in 2010.1 These patients were older than those who were treated (median age 77.0 versus 67.5 years, p=0.02) and had shorter median OS (1.8 versus 17.2 months, p<0.01), but otherwise had no statistically significant difference in baseline characteristics that were measured. Among the 36 cases in the study, 3 had a diagnosis of AML, NOS (pure erythroleukemia) and 33 had a diagnosis of acute erythroleukemia-erythroid/myeloid by 2008 WHO definitions. Using the 2016 WHO definitions, 29/36 (81%) cases would now be reclassified as MDS.
CONCLUSION: In this single-institution retrospective study, we found decitabine to be an attractive first-line option for patients with AEL, with high response rates and an OS rivaling cytarabine-based induction chemotherapy. Response rates and OS were numerically higher with decitabine compared with azaciditine; larger studies could be undertaken to see if there is a differential outcome between the two hypomethylating agents. The majority of patients in this study would be reclassified as MDS according to the WHO 2016 guidelines. Decitabine is indicated in MDS; however, the FDA approved regimen is not the 10-day regimen, which demonstrated excellent outcomes in this study. Thus, the 10-day decitabine regimen may be particularly beneficial in cases of MDS with erythroid hyperplasia (>50%) and a corrected blast percentage of ≥ 20%, cases that would have previously been diagnosed as acute erythroleukemia-erythroid/myeloid in the 2008 WHO, but may be overlooked in the 2016 WHO. Given the historically poor outcomes in patients with AEL, prospective analyses of 10-day decitabine in such patients are urgently needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal