Abstract
Allogeneic hematopoietic cell transplantation (HSCT) is a powerful consolidation option for acute myeloid leukemia (AML) patients (pts) in hematologic complete remission (CR). Disease recurrence after HSCT remains a major clinical problem & early identification of AML pts at risk of relapse is crucial to improve outcomes. High expression of the AML associated gene BAALC (Brain and acute leukemia, cytoplasmic) at diagnosis adversely impacts on outcomes in AML pts. Little is known about its prognostic capacity during disease course & as a marker of residual disease. Here we adopted digital droplet polymerase chain reaction (ddPCR) for absolute quantification of BAALC copy numbers in peripheral blood (PB) prior to HSCT in AML pts in hematologic CR.
We identified 82 AML pts with PB in first (60%) or second CR (23%) or CRi (17%) up to 28 days prior to HSCT available. Median age at HSCT was 63.9 (range 50.8-76.2) years (y). All pts received non-myeloablative (NMA) conditioning (fludarabine 3x30 mg & 2 Gy total body irradiation). At diagnosis, mutation status (mut) of the NPM1, CEBPA, IDH1, IDH2,& DNMT3A gene & presence of FLT3-ITD or FLT3-TKD were assessed. In pre-HSCT PB, absolute quantification of BAALC copy numbers was performed by ddPCR & results were normalized to ABL1 copy numbers.Additionally, absolute BAALC copy numbers wereassessedin PB of healthy controls (n=7) with a median age of 62.7 (range 39.6-82.0) y.
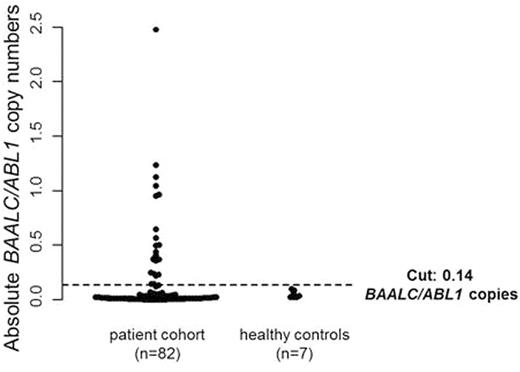
Pts were grouped according to the European LeukemiaNet (ELN) classification in 21% favorable, 23% intermediate-I, 24% intermediate-II, 23% adverse & 9% unknown. Pts & healthy control were evenly matched in age (P=1) & sex (P=1). BAALC/ABL1 copy numbers did not differ between AML pts at HSCT (median 0.03 [range 0.01-2.48]) & the healthy controls (median 0.04 [range 0.03-0.10], P=.34, Figure 1).
A cut-off point of 0.14absolute BAALC/ABL1 copies was determined using the R package 'OptimalCutpoints' & used to define pts with high (26%) & low (74%) pre-HSCT BAALC/ABL1 copy numbers. The copy number at this cut-off point was higher than the two-fold standard deviation over the median of the healthy controls (0.10 BAALC/ABL1).
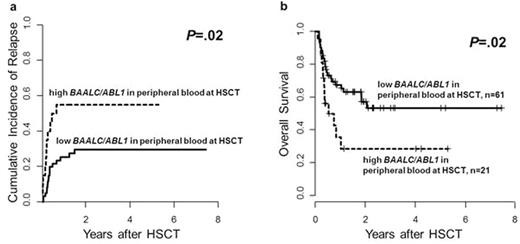
Pts with high & low pre-HSCT BAALC/ABL1 copy numbers did not differ significantly in pre-treatment characteristics (i.e. hemoglobin, white blood count, platelets, blasts in bone marrow or PB, ELN genetic group, FLT3-ITD, FLT3-TKD, NPM1, CEBPA, DNMT3A, IDH1 or IDH2 mut) or remission status at HSCT (CR1 vs. CR2 vs. CRi). However, pts with high pre-HSCT BAALC/ABL1 copy numbers had a significantly higher cumulative incidence of relapse (CIR, P=.02, Figure 2a) & shorter overall survival (OS, P=.02, Figure 2b). High pre-HSCT BAALC/ABL1 copy numbers especially impacted on CIR when we restricted our analysis to pts with normal cytogenetics (P=.003). In multivariate analysis for the entire cohort, high pre-HSCT BAALC/ABL1 copy numbers retained the prognostic impact on CIR (Hazard Ratio [HR] 3.6, Confidence Interval [CI] 1.6-8.2, P=.002) after adjustment for disease status at HSCT (P=.006) & the prognostic impact on OS (HR 2.2, CI 1.1-4.3, P=.02).
In conclusion, ddPCR is a feasible method for absolute quantification of BAALC copy numbers in PB, which may indicate residual disease burden in AML pts. High PB BAALC/ABL1 copy numbers (>0.14) in AML pts in hematologic CR at HSCT associated with higher CIR & shorter OS in univariate & multivariate models. AML pts with high PB BAALC/ABL1 copy numbers at HSCT should be closely monitored for relapse in the post-transplant period. In the future prospective studies will be required to validate the absolute PB BAALC/ABL1 copy number cut-off point & to evaluate whether AML pts with high BAALC/ABL1 copy numbersmight benefit from additional treatment before HSCT.
Poenisch:Mundipharma: Research Funding. Niederwieser:Amgen: Speakers Bureau; Novartis Oncology Europe: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal