Abstract
Introduction: Advances in chemotherapeutic regimens have improved pediatric ALL survival rates from less than 10% in the 1960s to approximately 90% currently (Hunger et al NEJM 2015). Asparaginase (ASP), as a component of the multi-agent chemotherapy regimen has become a cornerstone treatment for ALL. Pegaspargase (Oncaspar, PEG) is a pegylated asparaginase with sustained asparaginase activity, offering less frequent administration and associated with less treatment-related anxiety compared to native E.coli L-asparaginase (Place et al Lancet Oncol 2015). Due to the ample amount of data available for PEG, a systematic literature review and quantitative synthesis of evidence was previously performed to assess the relative benefit of PEG versus native ASP in newly diagnosed ALL patients in terms of event free survival (EFS) and overall survival (OS) (Kilpatrick et al ICPE 2016). The main objective of this investigation was to update the literature review by identifying additional relevant scientific clinical evidence of PEG in first line treatment of ALL in pediatric patients, and to expand the investigation to include adult patients.
Methods: All available evidence for newly diagnosed patients treated in pediatric and adult ALL protocols using PEG or native ASP was identified using a search algorithm. Randomized, observational and cohort studies were included. Outcomes were EFS and OS. Safety/immunogenicity was also examined. Feasibility was then explored in this order: direct comparison meta-analysis (MA) if at least two head-to-head trials were found, indirect comparison (network MA) if a common comparator could be identified or separate product MAs (pooled estimates) of PEG and native ASP using a random effects model. The individual product MA was analyzed for two important variables: risk category (standard & high risk) and by distinct age groups. Pooling was conducted, assuming normality, using the software Comprehensive Meta-Analysis, version 2.
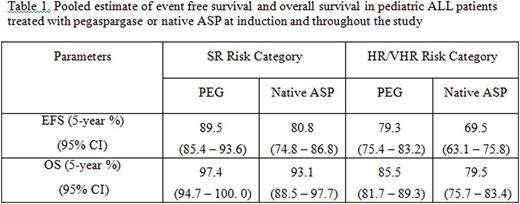
Results:There were 62 and 30 studies that met pre-specified inclusion criteria for abstraction for pediatric and adult populations, respectively. Some identified studies in both populations provided data for both asparaginases. Therefore, in pediatrics: 39 studies provided data for PEG and 41 for nativeASP, in adults: 10 studies provided data for PEG and 23 for nativeASP. Direct MA was not feasible as only one head-to-head study was identified. Also due to lack of common comparator, indirect MA was infeasible. Pooled estimate of EFS and OS data for pediatric ALL patients treated with PEG or native ASP is shown in table 1 below. Forest plot with pooled estimate of 5-year OS in adult ALL patients treated with PEG or native ASP is presented in figure 1, categorized by age group when available. Overall safety was consistent with product class for both asparaginases.
Conclusions: A systematic literature review and quantitative synthesis constitute an important approach to critically appraise relevant research. Consistent with published data, better outcomes were observed in pediatric than adults patients. The result from this updated research synthesisdemonstrates a positive effectiveness profile of PEG in the treatment of newly diagnosed ALL patients with less frequent administration.
Lebedinsky:Shire: Employment. Hale:Shire: Employment. Patel:Shire: Employment. Casamayor:Shire: Other: Vendor; Quintiles: Employment. Wijnands:Shire: Other: Vendor; Quintiles: Employment. Desai:Shire: Employment.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal