Abstract
Background: We previously reported on a novel bortezomib (bort)/tacrolimus(tac)/methotrexate (mtx) regimen with low rates of graft-versus-host disease (GVHD) and non-relapse mortality (NRM) and promising overall and progression-free survival (OS, PFS) in HLA-mismatched donor (MMD) reduced-intensity conditioning (RIC) hematopoietic stem cell transplantation (HSCT). To determine whether bort provided a meaningful improvement in outcomes, we undertook a prospective randomized controlled trial (RCT) of standard-of-care (SOC) tac/mtx versus 2 novel bortezomib-based GVHD regimens for RIC HSCT recipients lacking HLA-matched related donors (Clinical Trial ID: NCT01754389).
Intervention: The open-label phase II 3-arm 1:1:1 RCT enrolled adult hematologic malignancy patients aged 18-75 years. Conditioning was IV busulfan (0.8 mg/kg BID) and fludarabine (30 mg/m2 QD) from d-5 to -2. 8/8 matched unrelated donor (MUD) or 7/8 MMD T-replete PBSC grafts (≥ 2x106 CD34+ cells/kg) were infused on d0. GVHD regimens were: tac/mtx (arm A, SOC); bort/tac/mtx (arm B); and bort /sirolimus (siro)/tac (arm C) dosed as: bort (1.3 mg/m2 IV d+1, +4, +7), mtx (10 mg/m2 IV d+1, 5 mg/m2 d+3, +6, +11), siro (target trough level 5-12 ng/ml) and/or tac (target trough level 5-10 ng/ml) from d-3 with taper from d+100 and complete by d+180, as applicable per treatment arm. Primary endpoint was grade II-IV acute GVHD incidence by d+180. Secondary endpoints included NRM, relapse, PFS, OS and chronic GVHD at 1 year.
Patient and transplant variables: 138 evaluable patients with a median age of 64 years (range, 24-75), variable diagnoses (53 AML, 33 MDS, 20 NHL, 11 CLL, etc) and disease-risk indices (Low 14, Intermediate 96, High/Very High 28) were accrued between Jan 2013 and Nov 2015. They received 8/8 (98) MUD or 7/8 (40) MMD PBSC grafts. The treatment arms (A: 46; B: 45; C: 47) were balanced for pre-transplant variables, except for lower CMV seropositivity in arm C (78.3% vs. 77.8% vs. 53.2%, p=0.01). Median follow up in survivors was 15 months (range, 5.5-38).
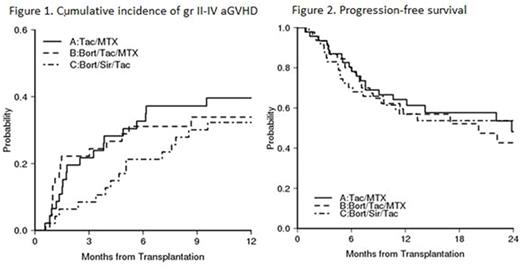
Outcomes: The regimens were well tolerated. No bort doses required omission or reduction. Grade 3-5 AE rates were similar across arms. TMA/HUS and VOD rates were not different (p=0.16, p=0.41, respectively). Median day +30 donor chimerism was ~96% (range, 42-100) across arms (p=0.84). The d+180 incidence of grade II-IV acute GVHD was similar overall across arms at 33% (A) vs. 31% (B) vs. 21% (C, p=0.65, Figure 1), but for the 8/8 MUD subgroup it was 33% (A) vs. 16% (B) vs. 19% (C) with a trend to significance for the bort-based regimens at 33% (A) vs. 17% (B+C, p=0.08). Across arms, the 1-year NRM incidence was 11% (A) vs. 15% (B) vs. 6.5% (C, p=0.43), and relapse was 24% (A) vs. 28% (B) vs. 36% (C, p=0.62). The 1-year incidence of extensive chronic GVHD was 39% (A) vs. 44% (B) vs. 48% (C, p=0.52). 1-year PFS was 64% (A) vs. 57% (B) vs. 57% (C, p=0.89, Figure 2), and OS was 72% (A) vs. 63% (B) vs. 70% (C, p=0.54).
Conclusions: 1. For 7/8 MMD RIC HSCT, adding bort does not provide benefit to SOC tac/mtx, which offers outcomes better than historically anticipated. 2. For 8/8 MUD RIC HSCT, adding bort may offer grade II-IV acute GVHD benefit, but direct randomization with an appropriately powered sample size would be required for confirmation.
Koreth:kadmon corp: Membership on an entity's Board of Directors or advisory committees; takeda pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; prometheus labs inc: Research Funding; amgen inc: Consultancy; LLS: Research Funding; millennium pharmaceuticals: Research Funding. Armand:Roche: Research Funding; Pfizer: Research Funding; Merck: Consultancy, Research Funding; Sequenta Inc: Research Funding; Infinity Pharmaceuticals: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding. Ritz:Kiadis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal