Abstract
Introduction: Storage of red blood cells (RBCs) for transfusion purposes is accompanied by a number of morphological and biochemical changes (storage lesions) that reduce post-transfusion survival/efficacy and increase risk for adverse reactions in the recipients. The clearance of altered and older RBCs from circulation is triggered by the clustering of Band 3, an aggregate state that is recognized by a low-affinity naturally occurring IgG antibody (Nab). Considering the key role of Band 3 in the maintenance of RBC structure and survival, elucidation of functional and structural modifications of Band 3 during storage should lead to new approaches aiming to improve RBC storage and post-transfusion viability.
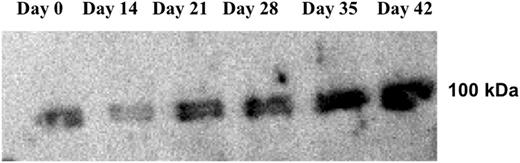
Results: Immunoblot analysis of RBC membrane proteins using an anti-phosphotyrosine antibody showed a progressive increase in the phosphorylation status of Band 3 during RBC storage (Figure 1).
In addition, using the quenching fluorescence of eosin-5-maleimide (EMA), we showed an increase of the mobile fraction of Band 3. These findings are consistent with previous demonstration that tyrosine phosphorylation of Band 3 reduces its affinity for ankyrin, leading to the release of the immobile fraction of Band 3 from the skeleton complex, and enhancement of the lateral mobility of Band 3 into the lipid bilayer. Immunoblot experiments using an antibody that specifically recognizes the clustered form of Band 3 revealed an increase of Band 3 cluster formation from the 28th day of storage. We also showed that the release of microparticles (MPs) that occurs during RBC aging increases from the 28th day of storage (Figure 2).
Finally, stopped-flow-based functional studies showed a decrease of the anion exchanger activity of Band 3 from the 28th day of storage.
Conclusion: Altogether, our results suggest that the 28th of storage represents a key moment for the molecular processes leading to irreversible lesions of RBCs and allow us to propose a new Band 3 phosphorylation/clustering-based mechanism of RBC aging.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal