Abstract
Introduction: Eltrombopag is an oral thrombopoietin receptor agonist, approved for the treatment of patients with cITP (persisting >12 months) aged ≥1 year, who are refractory to other treatments (eg corticosteroids, immunoglobulins). Pharmacokinetic studies of eltrombopag have demonstrated that patients of East Asian origin (eg Japanese, Chinese, Taiwanese and Korean) experience increased plasma exposure to eltrombopag compared to non-East Asian patients (predominantly Caucasian). As such, the recommended starting dose is 25 mg/day for East Asian patients, compared with 50 mg/day in non-East Asians. In the EXTEND (Eltrombopag eXTENded Dosing) study, all patients, irrespective of ancestry, received 50 mg/day starting dose that was subsequently adjusted to the platelet response. Unpublished anecdotal reports of platelet responses in East Asian patients from EXTEND describe lower doses of eltrombopag. Here, we examine the responses to eltrombopag in East Asian and non-East Asian patients who completed the EXTEND study.
Methods: Adult patients (≥18 years old) diagnosed with cITP who had completed a previous ITP study of eltrombopag were enrolled in EXTEND. All patients received eltrombopag starting at 50 mg/day, titrated to 25-75 mg/day or less often as required, based on individual platelet count responses (range ≥50-200x109/L). Maintenance dosing continued after minimization of concomitant ITP medication and optimization of eltrombopag dosing. Patients who received 2 years of treatment and transitioned off due to commercial availability of eltrombopag were considered to have completed the study. Patients could remain on study beyond 2 years until eltrombopag became commercially available. Here we describe the efficacy and durability of response in East Asian and non-East Asian patients. Analyses were conducted using the safety population, defined as all patients who had taken at least one dose of the study medication.
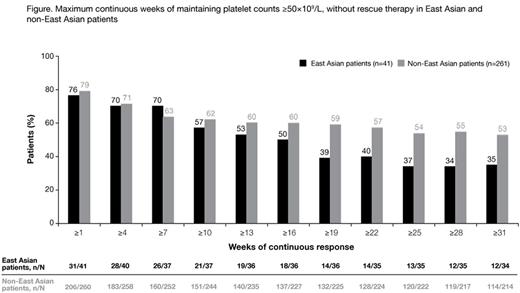
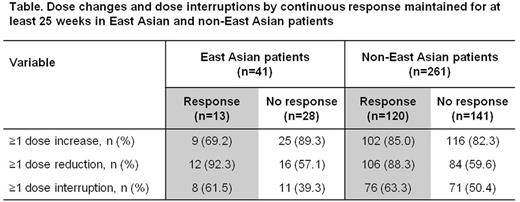
Results: Of 302 patients enrolled and exposed to treatment (median duration 2.4 years [range, 2 days to 8.8 years]), 41 (14%) were of East Asian origin. Mean average eltrombopag dose in East Asian and non-East Asian patients was 48.9 (range 4.2-74.9) mg/day and 50.4 (range 1.0-74.6) mg/day, respectively. Maintenance of platelet counts ≥30×109/L for at least 25 weeks was seen in 25/35 (71%) East Asian and 158/222 (71%) non-East Asian patients. In total, 13/35 (37%) East Asian patients and 120/222 (54%) non-East Asian patients maintained continuous platelet counts ≥50×109/L for at least 25 weeks, without rescue therapy (Figure). At the start of response, mean daily dose in East Asian and non-East Asian patients was 45.2 and 45.4 mg/day, respectively. The number of patients receiving dose adjustments according to platelet response was similar in East Asian and non-East Asian patients (Table).
Conclusions: Treatment with eltrombopag in East Asian and non-East Asian patients resulted in sustained platelet responses ≥30×109/L for at least 25 weeks in a similar proportion of patients. However, a higher proportion of non-East Asian patients achieved continuous platelet counts ≥50×109/L. Direct comparisons should be interpreted with caution because: a) of limited patient numbers in the East Asian group; b) the possible selection bias of patients entering the EXTEND study following completion of earlier eltrombopag studies, eg, primarily responders; and c) the absence of PK data from these patient groups. All patients received similar doses of eltrombopag irrespective of racial background, and dose modifications according to platelet responses were similar. Further investigations are ongoing to determine whether there were any differences in terms of safety and tolerability outcomes in East Asian and non-East Asian patients.
Wong:Bayer, Biogen-Idec, Bristol-Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Merck Sharp & Dohme, Novartis, Pfizer, and Roche: Research Funding; Biogen-Idec and Novartis: Membership on an entity's Board of Directors or advisory committees; Bayer, Biogen-Idec and Novartis: Consultancy. Bussel:Amgen, Novartis & GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingleheim, Prophylix Pharma, Protalex, Rigel Pharmaceuticals: Research Funding; Momenta Pharmaceuticals, Novartis, Prophylix Pharma, Protalex, Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; UptoDate: Patents & Royalties; Physicians Education Resource: Speakers Bureau. Saleh:GSK: Consultancy, Research Funding, Speakers Bureau. El-Ali:Novartis: Employment. Quebe-Fehling:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal