Abstract
Bone marrow/hematopoietic stem cell transplantation (BMT/HSCT) holds the remarkable ability to correct any blood or immune disease. Unfortunately, despite the tremendous potential of this procedure, BMT remains fairly limited in part due to the severe risks associated with the toxic conditioning regimens, such as irradiation and chemotherapy that are currently employed to enable donor HSC engraftment. Although significant work has been done to dose reduce the amount of these preparative agents, patients still experience many side effects including neutropenia/infections, anemia, mucositis, infertility, organ damage and secondary malignancies. Complete elimination of these toxic conditioning regimens could dramatically improve the safety profile of BMT and expand the potential applications to include many more non-malignant hematologic disorders, a wide variety of autoimmune disorders including diabetes, as well as facilitate solid organ tolerance.
We have previously shown that competition with host HSC limits donor HSC engraftment, and that in immunocompromised hosts antagonistic anti-ckit monoclonal antibodies deplete host HSC and are an effective and safe alternative conditioning approach (Czechowicz, Science 2007). However, this modality of conditioning is not effective in hosts with competent immune systems. To further understand efficacy of antagonistic anti-ckit conditioning, we tested its functionality in multiple strains of immunocompromised mice and show that inhibition of SCF signaling is not sufficient to deplete host HSC in mouse strains with competent B-cells or T-cells, and that the addition of these cells interferes with the ability of antagonistic anti-ckit antibodies to effectively condition. In an attempt to overcome this hurdle, wildtype mice were immune-depleted with a variety of regimens but none enabled antagonistic anti-ckit conditioning in the immunocompetent setting.
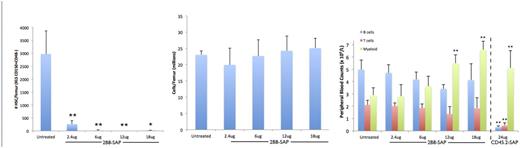
To strengthen the potency of anti-ckit mAbs we linked them to protein synthesis toxins, which when internalized by host HSC led to their rapid decline in vitro and in vivo. Administration of anti-ckit-saporin to wild-type mice resulted in >99% depletion of host HSC (Ckit+Lin-Sca1+CD150+CD48-), and lack of residual host HSC activity in the bone marrow was confirmed by CFC assays and competitive transplantation into lethally irradiated recipients. Interestingly, although ckit is expressed by a majority of HSPC, LT-HSC were most significantly affected and no cellularity changes in the bone marrow were observed. Uniquely this regimen was entirely non-peripheral blood ablative unlike other more broadly targeted conditioning regimens such as CD45 immunotoxins (Palchaudhuri, Nat Biotech 2016), and treated animals did not experience any significant depletion of myeloid, lymphoid, or erythroid cells.
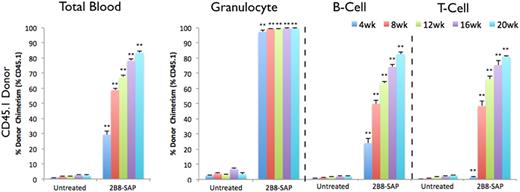
Treatment with anti-ckit-saporin effectively conditioned wild-type animals and near complete donor granulocyte chimerism was rapidly achieved post transplantation of whole bone marrow cells (99.54 ± 0.35 % vs. 6.79 ± 0.57 %, p<0.001), a >25-fold increase compared to unconditioned controls. Similarly, anti-ckit-saporin conditioning enable efficient engraftment of FACS purified donor HSC (Ckit+Lin-Sca1+CD150+CD48-). In both settings, donor HSC chimerism matched donor granulocyte chimerism further confirming replacement of host HSC. Importantly, host immunity was entirely intact in these animals throughout, with slower recalibration of the longer-lived immune cells given the lack of their direct depletion.
This work sets the stage for redefining the way BMT/HSCT is performed, as it opens up the possibility for entirely safe, quick and easy transplantation that potentially could be done in the outpatient setting with no perturbation to host immunity. Extrapolation of these methods to humans may enable efficient yet gentle conditioning regimens for transplantation, which is especially exciting in the gene-therapy settings where no immune suppression is required, allowing for simple, safe and curative treatment of a wide magnitude of grievous blood and immune diseases ranging from sickle cell to hemophilia to HIV. As multiple anti-ckit mAbs are currently in development and being tested in clinical trials, such an approach may be rapidly translatable to patients.
Czechowicz:Third Rock Ventures: Consultancy; Global Blood Therapeutics: Equity Ownership; Editas Medicines: Equity Ownership, Patents & Royalties; Decibel Therapeutics: Equity Ownership; Magenta Therapeutics: Consultancy, Equity Ownership, Patents & Royalties; Forty Seven Inc: Patents & Royalties. Palchaudhuri:Magenta Therapeutics: Employment, Equity Ownership, Patents & Royalties. Hoggatt:Magenta Therapeutics: Consultancy, Equity Ownership, Research Funding. Scadden:Teva: Consultancy; Apotex: Consultancy; Magenta Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Dr. Reddy's: Consultancy; GlaxoSmithKline: Research Funding; Fate Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Bone Therapeutics: Consultancy. Rossi:Magenta Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Intellia Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Moderna Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal