Abstract
Recently recognized but likely prevalent, mast cell (MC) activation syndrome (MCAS) presents as heterogeneous chronic multisystem polymorbidity of generally inflammatory ± allergic theme; severity can be disabling. MCAS features aberrant MC reactivity and constitutive MC activation with little MC accumulation [Afrin, Ann Med 48:190-201], distinct from mastocytosis (rare and notable for recurrent KIT codon 816 variants). Studies at Univ. of Bonn [Molderings, Scand J Gastroenterol 42:1045-53 and Immunogenetics 62:721-7] suggest most MCAS patients (pts) bear in peripheral blood (PB) MCs somatic, likely constitutively activating, non-codon-816 variants, and some splicing variants, in mRNA for KIT, the dominant MC regulatory gene (MCRG). Other studies show most mastocytosis, too, bears variants in many MCRGs beyond KIT [e.g., Soverini, Blood 126:4085], suggesting the same is likely in MCAS. Confirmation of MCRG variants in MCAS would spur new direction in research in MCAS and chronic inflammatory diseases (CIDs) possibly born of MCAS [Afrin, Transl Res 174:33-59]. We are studying (UMN IRB-approved protocol 2015NTLS023) mRNA for KIT and other MCRGs in PB MCs from MCAS pts and controls (ctls).
Methods: We extracted (Miltenyi CD117 immunomagnetic bead method per Bonn) PB MCs from 20 pts aged 18-50 diagnosed at UMN Jul-Dec 2015 with MCAS per criteria [Molderings, J Hematol Oncol 4:10] which in our experience (>1000 pts) reflects MCAS behavior better than other criteria [Valent, Int Arch Allergy Immunol 157:215-25]. We plan the same for 20 demographically matched ctls meeting rigorous good health criteria to exclude illness possibly from undiagnosed MCAS (9 ctls accrued so far, sequencing pending). Immunohistochemical staining with anti-human-c-kit antibodies (Thermo Scientific RB-9038-R7) confirmed extracts were pure bright CD117/c-kit-positive cells. mRNA was isolated (Qiagen RNeasy) and sequenced (Agilent HiSeq). Variant analysis of reverse-transcribed sequences from 38 genes (selected [Molderings, Crit Rev Oncol Hematol 93:75-89 and other reports] as regulating MC activation (receptor activation, signal transduction, cytokine production/release)) was performed via well-established GATK-based bioinformatic pipelines [Onsongo, BMC Res Notes 7:314], plus kallisto [Bray, Biotechnology 34:525-7] for finding RNA splice variants.
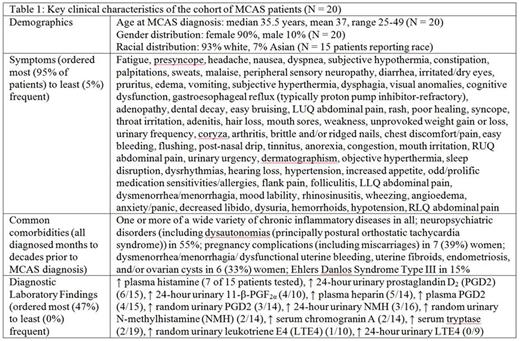
Results: Table 1 shows key pt characteristics (families similarly afflicted). Percentages of pts with elevations of various MC mediators tested in diagnosing MCAS were similar to prior report [Zenker, Blood 126:5174].
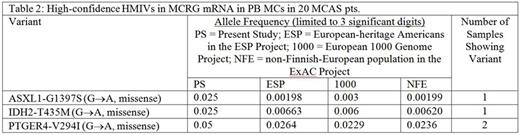
Exonic RNAseq showed many variants across many MCRGs in all 20 pts (median 11,276 variants per pt across studied genes; median 3,277 per pt were novel) but no recurrent variant signatures. Table 2 shows high- and medium-impact variants (HMIVs, i.e., frameshifts and missenses) found with high confidence (≥20 reads aligned to the region of the variant in all 20 pts); 8 KIT variants found were low impact (3' untranslated region). As ctls are not yet fully accrued, we show comparator allele frequencies from 3 reference datasets.
Other HMIVs were found with less confidence (<20 reads aligned to variant region in ≥1 pt) in ASXL1, CBL, DNMT3B, ETV6, EZH2, HNMT, IDH1, IL13, JAK2, KIT, KMT2A, KRAS, MS4A2, NLRP3, RASGRP4, SETBP1, SF3B1, TBXA2R, TET2, and TP53 (analysis ongoing). No HMIVs were found in ADGRE2, DNMT3A, HRH4, IL4, IL33, KITLG, MRGPRX2, NRAS, PDGFRα/β, RUNX1, SRSF2, SWAP-70, TNF, U2AF1, or VEGFA. We confirmed far greater expression of potentially more activating del(510-513) KIT splice variant in all pts.
Discussion: We did not find HMIVs in PB MC KIT mRNA in our pts (we soon will study another 20), but we found all bear many mRNA variants in many MCRGs, requiring further study for significance. Reference datasets may be inadequate comparators, skewed by unrecognized MCAS (suspected prevalence as high as 17% [Molderings, PLoS One 8(9):e76241]), so we are accruing rigorously healthy ctls for similar study. If our ctls are largely devoid of HMIVs in MCRG mRNA from PB MCs (as in [Immunogenetics, op. cit.]), a stronger case will be made that MCAS is primarily a clonal disease, if complicated, heterogeneous, and notwithstanding MC-targeted/activating autoimmunity. Further study of variants will include validation by direct DNA sequencing, characterization, and probing for recurrence in CID-specific MCAS subpopulations.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal