Abstract
Introduction :
Quantitative and qualitative analyses of hemoglobin (Hb) using protein approach allow in many cases to identify a variant present in a patient and thus to give a first counseling advice to couples displaying an abnormal Hb. This phenotype analysis requires the use of a battery of methods relying on different properties of proteins allowing a cross analysis enabling to decipher from the numerous variants described to date. However because of budget constraint, in an increasing number of laboratories, cation-exchange HPLC and capillary electrophoresis are the only two methods nowadays used for identification and quantification of a Hb variant. In many cases, these rapid phenotype analyses allow to predict the identity of the variant. But, this approach is misleading since several variants may display similar electrophoretic or chromatographic behavior and genetic factors may modify their expression level. This is important for variants which have mobilities close to that of Hb S, the most frequently observed variant. We describe herein a family with an α variant mimicking a heterozygous Hb S as well as for identification and quantification (fig 1).
Case report:
A newborn from a family of Azerbaijan was seen at the emergency pediatric care unit when 1 month-old because of worsening of an icterus. There was no specific disease history in the family and biological and clinical examination of the child led to the diagnosis of a moderate mixed bilirubin icterus (total bilirubin 150 µmol/L, conjugated bilirubin 6.1 µmol/L, with normal liver indices and blood count) without any severe symptom and a good clinical development. The child was not admitted . Because of her origins, she got a neonatal testing for Hb disease and the result showed the presence of a Hb variant. After genetic counseling, the identification of this variant was proposed to the family and done using CE-HPLC analysis and Capillary electrophoresis. It resulted in the finding in the father and the child of a variant compatible with Hb S as well for the mobility as for the percentage of the abnormal Hb component but negative according to the Itano solubility test. The blood samples were thus sent to our reference laboratory for identification of this unknown variant.
Methods :
Hb was studied using CE HPLC, capillary electrophoresis, isoelectrofocusing and Reverse Phase- HPLC. Sanger sequence identified the α-chain variant and polymorphic motifs located along α locus and specific to α 1 and α 2 gene.
Results :
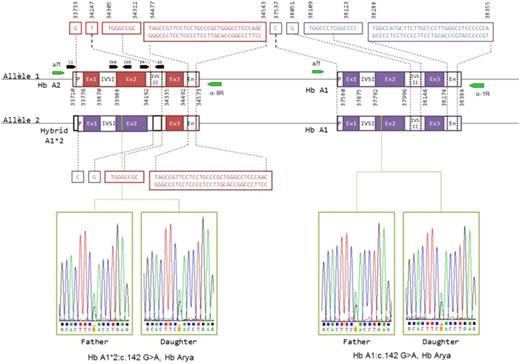
Hb Arya [α codon 47 Asp>Asn, HBA1( or HBA2):c.142G>A] was identified in the father and his girl and the unusual high level of expression was found to be related to a partial gene conversion from a1 to a2 making the patient and her father carrier of two copies of the αArya gene located in Cis (a2Arya- a 1Arya / a 2- a 1) (fig 2).
Conclusion :
Hb S phenocopy is not an uncommon finding and Hb variants were described to have chemical properties close enough to that of Hb S to be misleading when analyzed using only the "automated set" of method. This example is even more confusing because of the high percentage of the abnormal chain due to the gene conversion and militate against the ongoing budget driven simplification of Hb analysis. Proper diagnosis of Hb variant is not a simple process because methods used for diagnosis are based on electrical and chemical changes brought to the protein by the amino acid change or additional alteration. This can be mimicked or counter-balanced by another mutation. The only method to detect such artifacts is to use at least 3 methods based upon different electrophoretic or chromatographic protein characteristics and a solubility test. A positive solubility test or discrepancies in the result of the different tests used should trigger confirmation by Sanger sequencing of globin genes (β and/ or α globin).
High proportion for an α variant can be produced by several genetic configurations : homozygosity for the variant ( up to 40 %) or compound heterozygote with and α 1 thalassemia ( a-3.7, up to 30% ) however, these configuration are not transmitted as a dominant trait as in this family. A Gene conversion which make the two α genes of one chromosome carrier of the same variant allow that dominant transmission. Very few example are described but this example leads to speculate that conversion event between the two α genes could be more frequent than described and could account for unusual expression patterns.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal