Abstract
Background: The indications for treating children with immune thrombocytopenia (ITP) remain controversial. A valid and reliable bleeding assessment tool could assist in objectively quantifying bleeding and influence treatment decisions. Both the ITP Bleeding Score (IBLS) and the ITP-Bleeding Assessment Tool (BAT) have been developed to assess bleeding severity in ITP. The IBLS scores bleeding severity using an 11-item tool with grading from 0 to 2 and the 18-item BAT grades from 0 to 3 or 4. The BAT includes some types of bleeding not represented on the IBLS, such has intramuscular hematomas. To date, no data describe how these two measures compare when measuring bleeding associated with ITP.
Objective: To describe and compare bleeding as assessed by both the IBLS and BAT and to correlate bleeding severity with platelet counts in a cohort of children with ITP.
Methods: A longitudinal observational cohort of children ages >1 and < 18 years with ITP, were enrolled from 2013-2015 in the Pediatric ITP Consortium of North America ICON1 trial. All children were enrolled prior to starting a new second line monotherapy (not IVIG, steroids or anti-D). At enrollment, bleeding was assessed using the IBLS in all children. A subset of children also underwent a BAT assessment. Grades of bleeding were described and compared between tools and agreement in grading was assessed. Severity was correlated with platelet count using Spearman's correlation calculation.
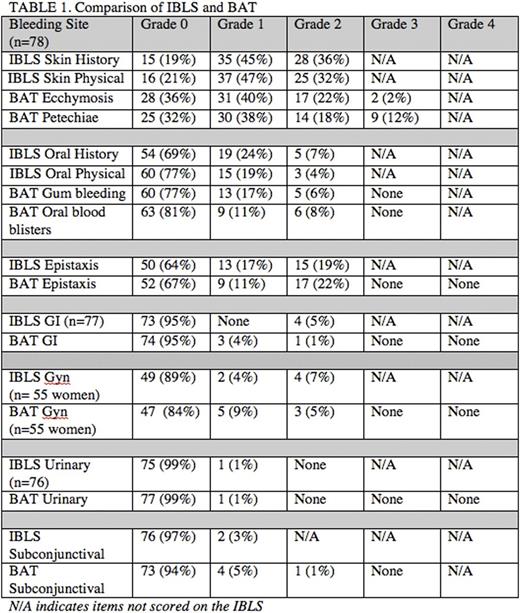
Results: 118 children were enrolled from 21 ICON centers. 54% had chronic ITP and the median age was 11.4y (range 1.2-17.8). The mean platelet count was 28 x 109/l (SD 57) and 88% had a baseline platelet count of <30 x 109/l. The burden of skin and oral bleeding was high. Table 1 compares bleeding scores for the 78 patients with both measures. Agreement for grades 0, 1, and 2 between the measures was highest for urinary bleeding (97%), gastrointestinal (95%), subconjunctival (95%), and epistaxis (91%). Agreement between IBLS oral bleeding by historyand BAT gum bleeding was 78% and with BAT oral cavity bleeding was 79%. IBLS oral bleeding by physical examination and BAT gum bleeding was 73% and BAT oral cavity bleeding was 79%. The lowest agreement was seen for skin manifestations. IBLS skin bleeding by history showed only 54% and 62% agreement with the BAT ecchymoses and petechiae items respectively. IBLS skin bleeding by physical examination showed 59% agreement with both the BAT ecchymoses and petechiae items. Grades 3 and 4 scores from the BAT did not provide additional information beyond the IBLS for most sites of bleeding. For sites included on the BAT but not represented on the IBLS, only 1 child had an intramuscular hematoma, 1 suffered an ocular bleed, and none experienced hemarthrosis. Bleeding from minor wounds and bleeding with tooth loss captured additional bleeding symptoms in 9 and 4 children, respectively. There were no episodes of pulmonary or intracranial hemorrhage in the cohort. Table 2 shows the correlation between bleeding severity and platelet count for all items on each measure.
Conclusion: The IBLS and BAT were similarly effective at identifying bleeding symptoms, although neither tool showed strong correlation with the platelet count. There were moderate correlations noted between skin bleeding scores and platelet counts for both tools. A major limitation of this comparison is the different definitions of bleeding severity between the two measures. For sites where the items were more detailed, agreement declined (i.e. adding specificity reduced generalizability). While no patients in our cohort exhibited significant grade 3 or 4 bleeding outside of skin findings, we conclude that in the setting of clinical trials, the ability to capture very severe bleeding might be an important distinction that supports using the more complicated BAT, but for clinical practice, a simplified assessment such as the IBLS may suffice.
Grace:Agios Pharmaceuticals: Other: Scientific Advisor, Research Funding. Neufeld:Novartis: Consultancy. Bussel:Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; UpToDate: Patents & Royalties; Protalex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genzyme: Research Funding; Ligand: Membership on an entity's Board of Directors or advisory committees, Research Funding; Symphogen: Membership on an entity's Board of Directors or advisory committees; Rigel Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shionogi: Membership on an entity's Board of Directors or advisory committees; Sysmex: Research Funding; Momenta Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Immunomedics: Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cangene: Research Funding; Prophylix Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Physicians Education Resource: Speakers Bureau; BiologicTx: Research Funding. Haley:CSL Behring: Honoraria; Baxalta: Membership on an entity's Board of Directors or advisory committees. Thompson:Celgene: Research Funding; ApoPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Mast: Research Funding; Amgen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Amgen: Research Funding; Baxalta (now part of Shire): Research Funding; bluebird bio: Consultancy, Research Funding; Baxalta (now part of Shire): Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; bluebird bio: Consultancy, Research Funding; Mast: Research Funding; ApoPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eli Lily: Research Funding; Eli Lily: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal