Abstract
Introduction: The Hestia criteria has been established as a highly sensitive prognostic tool used by clinicians to identify newly diagnosed pulmonary embolism (PE) patients from the general population at low vs. high risk of early (30 day) mortality. Font et al. applied similar risk stratification criteria in a population restricted to PE patients with cancer. We evaluated the performance of the generic Hestia tool and cancer-specific criteria by Font et al. for predicting 30-day post-PE mortality in patients with active cancer.
Methods: We identified consecutive, adult, objectively confirmed PE patients with active cancer presenting to the emergency department at our institution from 11/2010-1/2014. We calculated the proportion of patients categorized as low-risk by each set of criteria and determined the accuracy of the criteria for predicting 30-day all-cause mortality. Mortality was determined through Social Security Death Index searches.
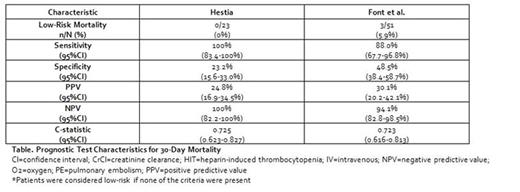
Results: A total of 124 patients with PE and active cancer (mean age 66.2 years, 46.0% with concurrent deep vein thrombosis, 49.2% with metastatic disease and 46.8%, 16.9% and 10.4% receiving chemotherapy, radiation or both, respectively) were included. Mortality at 30-days occurred in 25 (20.2%) patients. The Hestia tool had a sensitivity of 100% but classified <19% of patients as low-risk; while criteria developed by Font et al. had a sensitivity of 88.0% and classified 41.1% of patients as low-risk (Table). The most common reason patients were classified as high-risk by Hestia but not the criteria from Font et al. was the requirement for oxygen (O2) therapy to maintain an O2 saturation >90% for >24 hours (n=19).
Conclusion: In this external validation study, both the generic Hestia tool and cancer-specific Font et al. risk stratification criteria displayed acceptable sensitivity. Compared to Hestia, the Font et al. criteria classified two times as many patients as low-risk. Larger, prospective studies are needed to confirm our results.
Coleman:Janssen Pharmaceuticals: Consultancy, Research Funding; Bayer Pharmaceuticals AG: Consultancy, Research Funding; Boehringer-Ingelheim Pharmaceuticals, inc.: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal