Abstract
Introduction
Treatment outcomes for adult patients with relapsed ALL are limited. Allogeneic hematopoietic cell transplantation (HCT) is the only curative option in a small subset of patients. We sought to investigate the optimal extent of therapy required prior to proceeding to transplant in second complete remission (CR2).
Methods
126 consecutive patients with ALL in CR2 underwent HCT at MD Anderson Cancer Center between January 2004 to December 2015. The patient and transplant characteristics are described in table 1. The probabilities of outcomes were calculated with the Kaplan-Meyer method. Variables found to be significant at the p<0.1 level were included in a Cox regression multivariate model. Relative risks (RR) are reported with 95% confidence intervals (CI). All p values are two sided.
Results
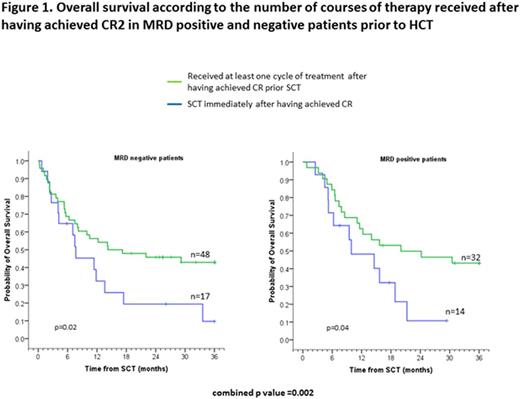
Median follow-up was 38.4 months (range 6-125). The 3-year overall survival (OS), progression-free survival (PFS) and transplant-related mortality (TRM) were 34.6%, 29.5% and 33.2%, respectively. Eighty-three patients received at least one course of chemotherapy after achieving CR2 (median 2, range 1-6). These patients had significantly better OS, PFS and relapse rate than the 34 patients that went to transplant immediately after achieving CR2. Namely, 43.8% vs 7.6% (p=0.002), 39.4% vs. 5.1% (p=0.002) and 37.9% vs. 89.5% (p<0.001), respectively. Both groups had comparable TRM (Table 1). Interestingly, these differences were independent of the patient's minimal residual disease (MRD) status prior to transplant (Figure 1). Similar results were found when we limited the analysis to the 96 patients who achieved CR2 with the first line of salvage therapy. OS in this group was 46.0% vs. 9.4% (p=0.009) for patients who required additional lines of salvage chemotherapy to achieve CR2.
Patient's age, performance status, comorbidity index and graft source were also predictive for outcome. In multivariate analysis receiving at least one additional course of chemotherapy after achieving CR2 (RR=0.53, CI=0.31-0.91, p=0.02), age>45 (RR=2.06, CI=1.20-3.53, p=0.009), alternative graft sources (RR=1.81, CI=1.09-2.97, p=0.02) and Karnofsky <90% (RR=1.68, CI=1.01-2.79, p=0.04) were independent predictors for OS. Receiving at least one additional course of chemotherapy after achieving CR2 (RR=0.52, CI=0.31-0.87, p=0.01), age>45 (RR=1.92, CI=1.15-3.20, p=0.01) and Karnofsky <90% (RR=1.86, CI=1.14-3.03, p=0.01) were independent predictors for PFS.
Conclusion:
The outcome of patients with relapsed ALL who proceed to HCT immediately after achieving CR2 is dismal. Our data supports the notion that patients should receive at least one additional course of treatment after achieving CR2 prior to transplantation, regardless of MRD status. However, our observations must be interpreted with caution as this was not an intent to treat analysis so we could not adjust for loss of patients prior transplantation. Further analysis on an intent to treat basis is underway.
Ciurea:Spectrum Pharmaceuticals: Other: Advisory Board; Cyto-Sen Therapeutics: Equity Ownership. Jabbour:Pfizer: Research Funding; BMS: Consultancy; ARIAD: Research Funding; Pfizer: Consultancy; Novartis: Research Funding; ARIAD: Consultancy. Kantarjian:BMS, Pfizer, Amgen, Novartis: Research Funding. Champlin:Intrexon: Equity Ownership, Patents & Royalties; Ziopharm Oncology: Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal