Abstract
Introduction: Myeloablative conditioned (MAC) allogeneic hematopoietic cell transplantation (alloHCT) with ex-vivo CD34+ selection results in favorable disease control and lower incidence of graft-versus-host disease (GVHD) without the need for pharmacologic immunosuppression. However, detailed analyses of post-HCT toxicities with this platform are limited. We compiled all high-grade toxicities in long-term survivors of ex-vivo CD34+ selected alloHCT with the goal of identifying areas of potential toxicity mitigation by improved patient (pt) selection and post-HCT management.
Methods: We retrospectively collected all grade ≥ 3 adverse events by CTCAE v.4.0 of 131 adults who underwent a MAC alloHCT with ex-vivo CD34+ selection (CliniMACS® CD34 Reagent System) as GVHD prophylaxis between 2006 - 2012 and who were alive without relapse/progression at 1 year. Individual toxicities were organized into 17 organ-based groups and 1 toxicity per group per specified time period after the 1-year landmark was used for statistical analyses. Cox regression was used to compare the risk of toxicities across pt and treatment characteristics, overall survival (OS) and non-relapse mortality (NRM).
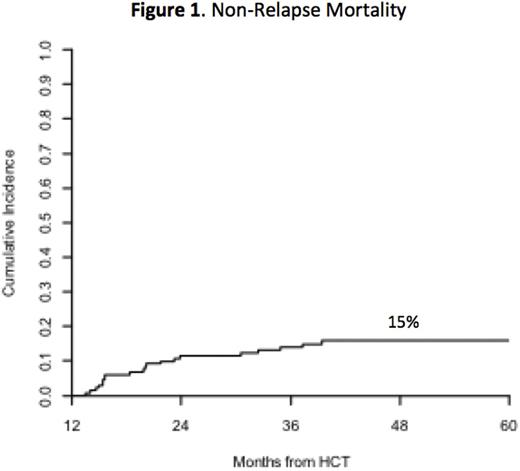
Results: We included 97 pts with AML/ALL/MDS (74%), 15 pts with multiple myeloma (11%), and 19 pts with other histologies (15%). Median age was 54 (19-72), and 46 pts were ≥ 60 years old. MAC was chemotherapy- and total body irradiation-based in 71% and 29% of pts, respectively. Median HCT-Comorbidity Index (HCT-CI) was 2 (0-10). Allografts were HLA MRD or MMRD in 52 pts (40%) and MUD in 53 (40%) or MMUD in 26 (20%). The overall incidence of grade 2-4 acute GVHD at day 100 was 10%, and only 4 of 13 pts had grade 3-4 GVHD. The incidence of grade 2-4 late acute GVHD was 14%. Median follow-up of survivors was 36 months post-landmark. During the study period, 29 pts died: 9 (7%) of relapsed disease and 20 (15%) of NRM (Figure 1). At 4 years, OS and progression-free survival (PFS) for the cohort were 77% and 70%, respectively (Figure 2A). A HCT-CI ≥ 3 was associated with poorer OS [HR 2.5 (95% CI: 0.9-6.6), p=0.036] and higher NRM [HR 5.3 (1.2-23.1), p=0.004].
The most common toxicities occurring 1-year post-HCT (N=285) were: infectious (24%), hematologic (20%), metabolic (17%), hepatic (8%), cardiovascular (6%), pulmonary (4%), and other (20%). The median number of toxicities within the first year was 7. Pts with > 7 toxicities within the first year had a 4-year OS of 67% versus 86% [HR 3.2 (1.4-7.2), p=0.006] (Figure 2B) and higher NRM [HR 4.8 (1.6 to 14.4)], p=0.005. Having > 7 toxicities within the first year correlated with more hematologic [HR 2.77 (1.5-5.2)], p=0.001, infectious [HR 3.6 (1.7-7.4)], p=0.001 and metabolic [HR 3.4 (1.5-7.6)], p=0.004 toxicities. Table 1 shows the toxicity groups whose development was associated with poorer OS and higher NRM.
Grade 2-4 GVHD in the first year was associated with worse OS [HR 3.5 (1.7-7.3), p=0.001] and higher NRM [HR 5.9 (2.4-14.3), p=<0.001]. Pre-HCT ferritin > 1000 ng/mL increased the risk of NRM [HR 3.6 (1.3-10.3), p=0.032], whereas albumin > 4.0 reduced the risk of NRM [HR 0.4 (0.2-0.9), p=0.03]. Pre-HCT absolute lymphocyte count (ALC) > 0.5 K/mcL was associated with improved OS [HR 0.3 (0.1-0.7), p=0.004], decreased risk of NRM [HR 0.3 (0.1-0.8), p=0.015] and a reduced risk of hematologic [HR 0.3 (0.14-0.6), p=0.001], infectious [HR 0.4 (0.2-0.9), p=0.023] and metabolic [HR 0.4 (0.14-0.96), p=0.04] toxicities. Age, gender, disease, HLA match, MAC type, CMV status, busulfan dose, and use of Palifermin were not associated with the risk of toxicities, OS or NRM.
Conclusions: One-year survivors of ex-vivo CD34+ selected MAC alloHCT have excellent OS and a toxicity burden comparable to unmanipulated MAC alloHCT based on BMT CTN data. The number and type of toxicities in the first year, HCT-CI, and development of GVHD predict for higher NRM and poorer OS. Pre-HCT ALC and albumin are biomarkers for less toxicity, lower NRM, and improved OS and may contribute to previously validated pre-HCT prognostic tools. These analyses have identified areas of potential investigation to further mitigate post-HCT toxicity and reduce NRM. Moreover, these data may serve as a guide for health providers of pts enrolled on the pivotal Phase III BMT-CTN 1301 PROGRESS II trial of calcineurin inhibitor-free interventions for GVHD prevention.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal