Abstract
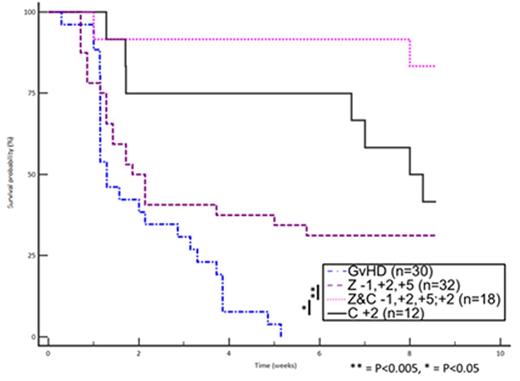
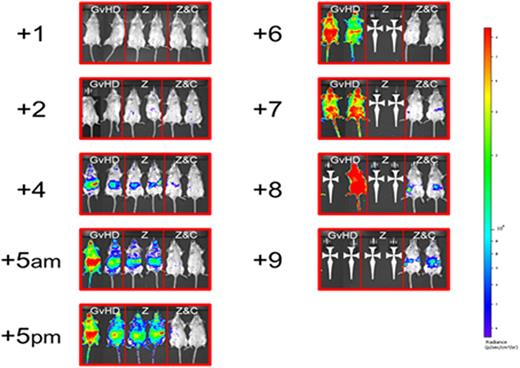
The concept of suppressing dendritic cells (DCs) to prevent graft-versus-host disease (GvHD) following allogeneic hematopoietic stem cell transplantation (HSCT) has been rejuvenated since the introduction of proteasome inhibitors into clinical practice. Although early post-transplant administration of proteasome inhibitors ameliorates GvHD, delayed administration has been associated with alloreactivity-dependent gastrointestinal toxicity. This phenomenon is associated with a surge in interleukin-1β (IL-1β) and acceleration in donor T cell expansion. To address this issue, we sought to investigate whether in vivo depletion of donor T cells by concomitant administration of post-transplant cyclophosphamide (C) could overcome this phenomenon. Ten-week old BALB/c mice were irradiated (10 grays in two fractions) on day -1 and transplanted with 5x106 BM cells from C57BL6 mice. GvH inocula consisted of 5x106 splenocytes from C57BL6 (some post-CFSE labeling) or B6;FVB-PtprcaTg(CAG-Luc,-GFP)L2G85Chco Thy1a/J (ubiquitously transgenic for firefly luciferase for bioluminescence imaging (BLI). All mice were purchased from Jackson Laboratory and kept in a pathogen-free environment. Mice were untreated or received ixazomib (Z) (30μg, subcutaneously) on days -1, +2, and +5, C (1 mg intraperitoneally) on day +2, or both Z and C according to the same schedule. Mice were monitored for weight, GvHD score, and survival or sacrificed on day +6 for cytokine measurement and splenic cell counts. For BLI, mice receiving transgenic splenocytes were injected with 150mg/kg of D-Luciferin intraperitoneally and imaged 25 minutes later. GvHD worsening and sudden death occurred in a fraction of mice receiving Z following day +5 injection. The phenomenon was completely prevented by the addition of C. Overall, the group receiving both Z and C had significantly improved weight, GvHD score, and survival when compared to the mice left untreated or receiving either drug alone (figure 1). The addition of C to Z prevented IL-1β increase, was associated with suppression of tumor necrosis factor α (TNFα), and resulted in a profound depletion of splenic total and donor CD4+ cells. Furthermore, proliferating cells (CFSE low) were preferentially depleted as opposed to quiescent cells (CFSE high). BLI imaging showed an increase in signal intensity shortly after the administration of Z on day +5. On the other hand, the mice receiving C in addition to Z exhibited significantly lower donor T cell proliferation when compared to the other groups (figure 2).
These findings suggest that C can prevent the phenomenon of GvHD acceleration associated with the extended administration of proteasome inhibitors, suppress cytokine production, and effectively reduce splenic total and donor CD4+ cell expansion with preferential depletion of proliferating as opposed to quiescent cells. The combination of Z and C seems a promising duplet for the prevention of GvHD. When Z is administered late following allogeneic HSCT for the prevention of disease relapse, the addition of C should be considered in order to avoid any unwanted aggravation of alloreactivity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal